Preparation and Standardization of 0.1 M Sodium Hydroxide (NaOH) in the Laboratory.
Reagents:
| 1 | Sodium hydroxide |
| 2 | Potassium hydrogen phthalate |
| 3 | Phenolphthalein solution. |
Chemical Reaction
Principle: In this process, we will directly measure the strength of potassium hydrogen phthalate by using sodium hydroxide. To find the endpoint, we’ll use phenolphthalein as an indicator. Let’s understand the reaction involved in this titration
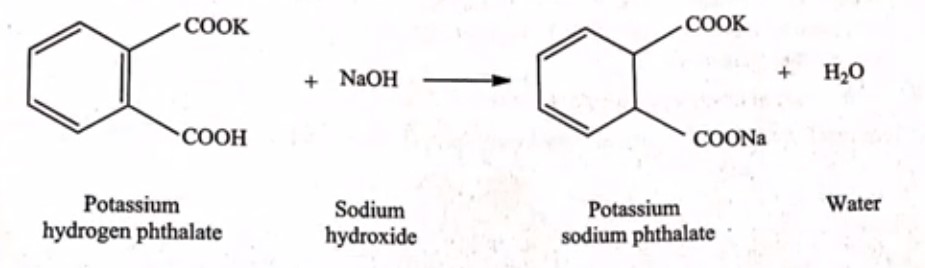
Example for factor calculation for standardization of 0.1 m NaOH:
Na=23
O=16
H=1
Total= 40 gm.
Preparation of 0.1 M Sodium Hydroxide (NaOH)
Step 1: Dissolve 4 g of sodium hydroxide in 1000 ml of distilled water.
Standardization of 0.1 M Sodium Hydroxide
Step 1: Clean and dry all glassware using standard laboratory procedures.
Step 2: Take 500 ml of an unknown stock solution of NaOH in a clean and dry beaker.
Step 3: Rinse the burette with distilled water, and then pre-rinse it with a portion of the NaOH solution before filling it up for the titration. Pre-rinsing ensures that the burette contains only the desired NaOH solution, without any dilution or contamination. To do this, add about 10 ml of the NaOH solution to the clean burette. Slowly tilt the burette on its side, allowing the liquid to run out from the top. Rotate the burette during this process to ensure the solution covers all sides. Pour the rinse into a waste beaker. Repeat this process with another portion of the NaOH solution.
Step 4: Accurately weigh about 1 g of potassium hydrogen phthalate powdered and heated previously at 120°C for 2 hours. Transfer it to a conical flask.
Step 5: Add 15 ml of distilled water to the conical flask and sonicate it on a sonicator for 5 minutes to dissolve properly.
Step 6: Add 2 drops of phenolphthalein indicator to the conical flask.
Step 7: Fill the burette with the unknown stock NaOH solution.
Step 8: Start the titration with the NaOH solution until you reach the endpoint. In the first titration, add 0.50 ml portions of NaOH solution from the burette while swirling the flask. The endpoint is indicated by a temporary appearance of a pink color that fades when the solution is swirled for up to 10 seconds. A pink color that persists for more than 30 seconds signals the actual endpoint.
Note: The solution may lose its color after 30 seconds or more, but it is not considered.
Step 9: The first titration gives an approximate idea of the amount of NaOH needed to neutralize the potassium hydrogen phthalate.
Step 10: Record the reading of the burette.
Step 11: Repeat the titration three times to obtain precise readings. For this, quickly add about 70% of the required NaOH and then, near the end, add NaOH one drop at a time until the endpoint is observed.
Step 12: Take the mean of the three titration readings and calculate the molarity of NaOH.
Step 13: The equivalent factor of potassium hydrogen phthalate for 0.1 M NaOH is 0.020422.
How to Make a 0.1M NaOH Solution from 1M NaOH:
A 0.1M NaOH solution is less strong than a 1M NaOH solution, about 10 times less concentrated. Follow these steps:
- Get a clean and dry 1000ml volumetric flask.
- Pour 10 ml of 1M NaOH solution into the 1000 ml volumetric flask.
- Add distilled water to fill the flask up to 1000ml, and mix it well.
Calculation of 0.1 M Sodium Hydroxide

Related: Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
Important FAQs
Ans: To prepare a 100ml solution of NaOH with a concentration of 0.1 moles per liter (M), you will need to follow these steps:
• Find the molar mass of NaOH, which is 40g.
• Use the formula for molarity (M): Molarity (M) = Volume of solution in liters (V) / Number of moles of solute (n). We want a 0.1M solution, and we have a volume of 100 ml, which is 0.01 liters (0.01L).
• To calculate the number of moles (n), rearrange the formula: n = M x V = 0.1 x 0.01 = 0.01 moles.
• Now, we need to figure out the mass of NaOH required to make the 100ml 0.1M solution. The mass is given by: Mass = n x Molar mass = 0.01 x 40 = 0.4g.
• Take 0.4 grams of NaOH and put it in a beaker.
• Gradually add a small amount of water to dissolve the NaOH.
• Continue adding water until the total volume reaches 100ml.
Ans: Because we require those indicators that give color in the base medium

