The Cleaning Validation Protocol is an essential part to execute the cleaning validation process.
| Title of Contents |
| Objective |
| Scope |
| Responsibility |
| Procedure |
| Sampling Plan |
| Sampling Points |
| Test/Method |
| Acceptance Criteria |
| ANNEXURE A – Cleaning Validation Test Request Form |
| ANNEXURE B – Cleaning Validation Test Report |
| ANNEXURE C – Cleaning Validation Test Report |
1.0 OBJECTIVE To assure that the cleaning procedure for Equipment removes residues to the extent of compliance with the pre-determined acceptance level. (Sifter As an Example)
2.0 SCOPE: This protocol applies to the Pharmaceutical Manufacturing Facility.
3.0 RESPONSIBILITY: It is a joint responsibility of the Quality Control, Production, and Quality Assurance departments. The detailed responsibility matrix is given below
- Protocol Preparation- Executive QA
- Protocol Approval- Head QA
- Protocol Execution – Head Production
- Sampling as per Protocol – Executive QA
- Testing as per Protocol- Executive QA
- Test result view And approval- Manager QA
- Validation Data Compilation- Executive QA
- Validation final approval- Head QA
4.0 PROCEDURE
Clean the equipment as per SOP “Cleaning of Equipment”.
Sample from the specified sampling points (6.0) as per the sampling plan (5.0).
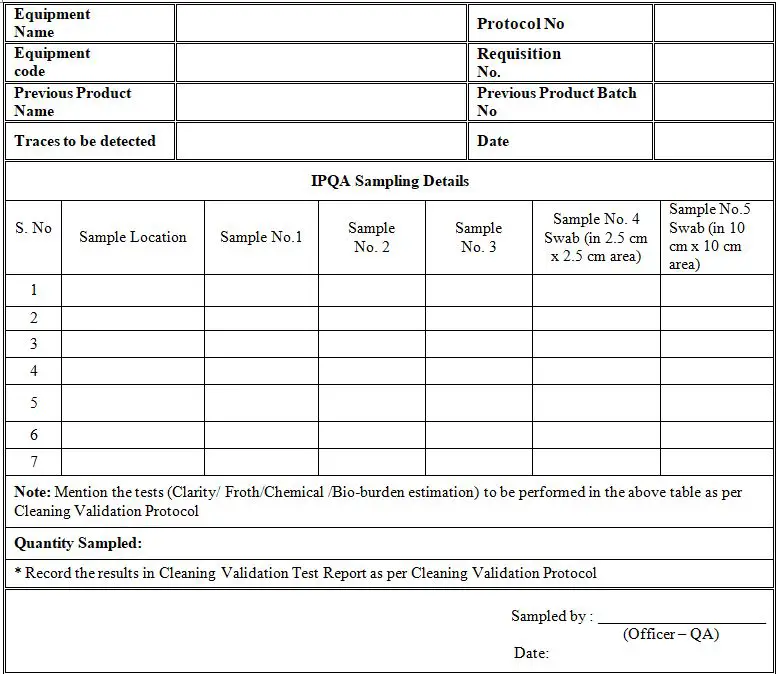
5.0 SAMPLING PLAN
- Sample from first rinse (after washing with raw water).
- Sample from second rinse (after cleaning with 0.1% liquid soap solution and raw water).Sample from final rinse of purified water.
- Swab (in 2.5 cm x 2.5 cm area) for chemical analysis.
- Swab (in 10 cm x 10 cm area) for bio-burden.
6.0 SAMPLING POINTS
- Upper Hopper
- Sieve used for the batch
- Powder discharge assembly
Note: Sampling points may differ from equipment to Equipment (We used Shifter for Reference)
7.0 TEST / METHOD
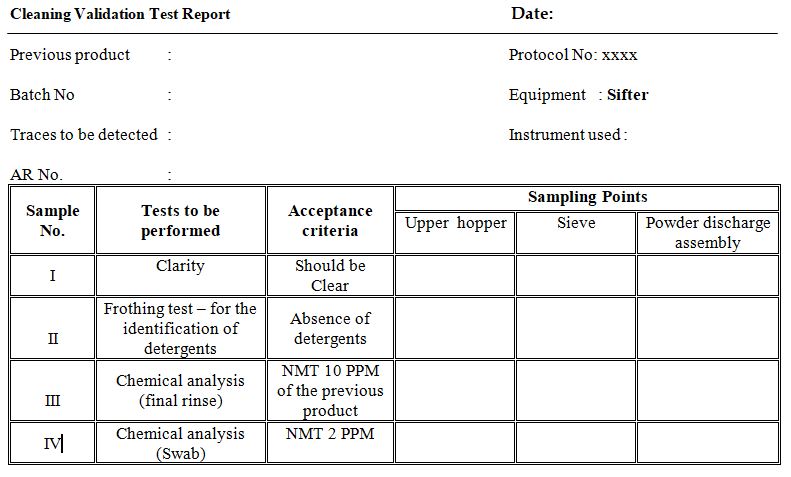
- Inspect visually for the absence of previous products in the equipment (sample I)
- Determine the absence of detergents by the froth method (sample-II ).
- Determine the concentration of active ingredient of the previous product in the final rinse (sample III).
- Determine the concentration of active ingredient of the previous product in the swab (sample IV).
- Report and record the above test (1,2,3 and 4) results in ANNEXURE – B
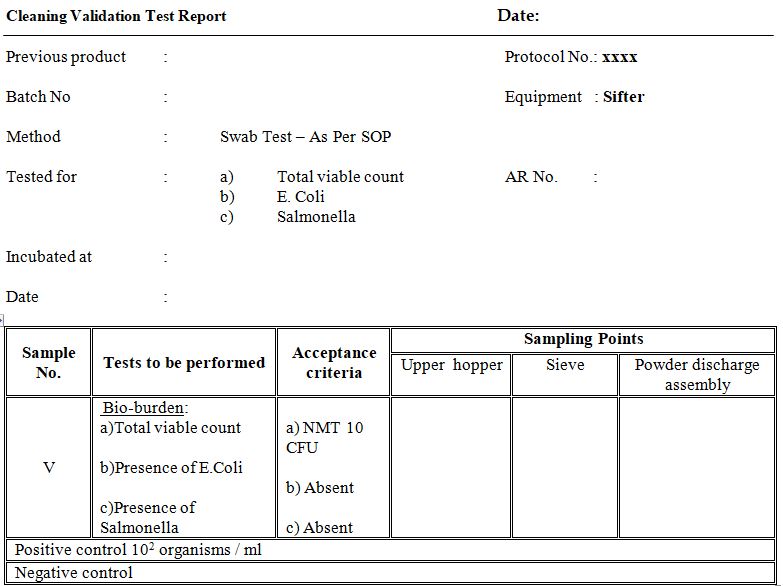
- Determine the bio-burden(sample V) and record the results in ANNEXURE– C
8.0 ACCEPTANCE CRITERIA
- No traces of the powder shall be visible on any part of the machine (sample-I)
- Detergent should not be identifiable by frothing test. (Sample-II).
- The concentration of the previous product should not be more than 10 PPM in the final rinse i.e. (sample III)
- The concentration of the previous product should not be more than 2 PPM in the swab (i.e. sample IV)
- Bio-burden limits are as below (sample V)
- Total CFU – NMT 10 CFU
- E. Coli -absent
- Salmonella – absent
Annexure- A (Cleaning Validation Test Request Form)
Annexure- B (Cleaning Validation Test Report)
Content of ___________________________in PPM
Area of sample x weight taken x dilution factor x 1000
Area of std. 100
Note: Attach calculation sheet and chromatogram.
Conclusion / Recommendations:
_______________________________________________________________________________________
Tested by: ______________ Approved by: ________________
(Analyst – QC) (Manager – QC)
Date:: Date:
Annexure – C (Cleaning Validation Test Report)
Analyzed by: _______________ Approved by: ________________
(Microbiologist) (Manager – QC)
Date: Date:

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
