Learn about Standard Operating procedures for Storage and Movement of unlubricated granules / Transfer including their Purpose, Scope, procedure for Storage of Unlubricated / Graded Granules, transfer of Unlubricated Granules to Blender Room, Precautions, and Annexures.
1.0 Purpose: 1.1 To lay down the Standard Operating Procedure for storage and movement of unlubricated granules and their transfer to the blender room.
2.0 Scope: 2.1 This SOP is applicable to the storage and movement of all unlubricated granules and their transfer to the blender room in the Manufacturing Department of the Pharmaceutical industry.

3.0 Responsibility
3.1 A trained worker / Operator shall be responsible for the movement of material in the Production Area as per this SOP.
3.2 Officer Production is responsible for the implementation of this SOP.
3.3 Head Production is responsible for ensuring overall compliance with this SOP.
4.0 Procedure
4.1 Storage of Unlubricated / Graded Granules:
4.1.1 Receive the Unlubricated Granules in closed Conta-bin/ In-process containers in double-lined fresh polybags, along with sifted lubricant in double-lined fresh polybags in HDPE drums.
4.1.2 Ensure that the proper Status Label is affixed on Conta Bin/ In-process containers of Unlubricated granules and on the HDPE drum containing the sifted lubricant.
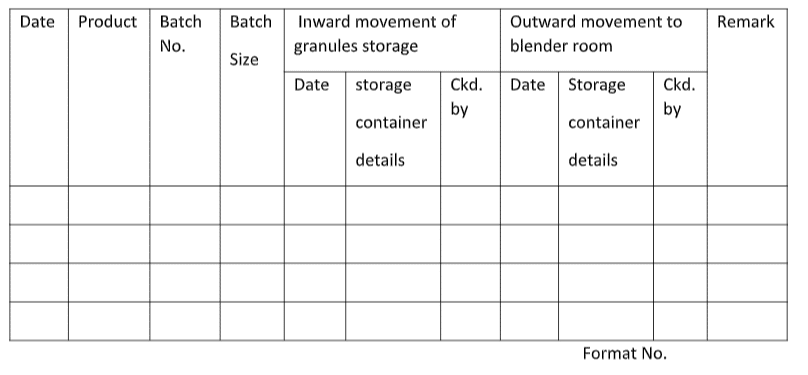
4.1.3 Record the inward details of Unlubricated Granules as per Annexure I “Un-lubricated Granules movement Record.”
4.2 Procedure for transfer of Unlubricated Granules to Blender Room:
4.2.1 Ensure the cleanliness of the Blender Room and its Line Clearance from IPQA before transferring the Unlubricated granules to the Blender Room.
4.2.2 Ensure the correct product name and B.No on unlubricated granules and lubricants to be sifted before transferring it to a blender room.
4.2.3 Transfer the Unlubricated granules and record their outward movement as per Annexure-I “Un-lublicated Granules Movement Record.”
Precautions: Ensure proper handling of materials during the transfer process. Also, check persons who are involved in the materials handling follow the Gowning procedure. Properly check the cleanness of the Conta bin wheels while transferring them to a blender room.
5.0 Abbreviations
No. Number
Pg. Page
SOP: Standard operating procedure
HDPE: High-density polyethylene
B.No. Batch Number
IPC: In-process container
Annex. Annexure
ANNEXURE-I: Unlublicated Granules Movement Record

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
