ICH Q10 guidelines basically mention the effective Pharmaceutical quality system (PQS). the pharmaceutical quality system is based on the ISO quality system including good manufacturing practice (GMP) regulations.
Relationship Between ICH Q8, ICH Q9 and ICH Q10
Before the ICH Q10, ICH Q8 (Pharmaceutical development), and ICH Q9 (Quality risk management) were implemented throughout the different stages of the pharmaceutical product lifecycle.
The purpose of ICH Q10 is not to introduce any new beyond what is required by the current regulations. Therefore, the information in ICH Q10 that It is optional to add to the existing regional GMP criteria.
ICH Q10 describes a system to improve the quality and accessibility of medications globally for the benefit of public health. The ICH Q10 should be used across the whole lifecycle of the product to encourage innovation, promote ongoing improvement, and strengthen the connection between pharmaceutical development and manufacturing activities.
Related: ICH Guidelines
The objective of ICH Q10
ICH Q10 Basically approaches the three Objectives:
- Achieve Product realization
- Establish and maintain a state of control
- Facilitate continual improvement
1. Achieve Product Realization
The purpose of the ICH Q10 is to establish a system to maintain the quality of products to meet the patient’s needs, the healthcare workforce, and regulatory agencies with compliance.
2. Establish and Maintain a State of Control
Developing and utilizing efficient monitoring and control systems for process performance and product quality will ensure the processes’ continuing suitability and capability.
It may be helpful to identify the monitoring and control systems using quality risk management.
3. Facilitate Continual Improvement
Continuous improvements in the product, and the process lead to the enhancement of the product quality system. quality management system plays a role in identifying and ordering areas for continual improvement.
1. What does ICH Q10 look for in Management Responsibilities?
The management is an essential part of maintaining a commitment to product quality and performance of the PQS.
⦿ Management: The senior management’s ultimate duty for ensuring that a strong pharmaceutical quality system is in place to meet the quality objectives as well as that roles, responsibilities, and authorities are established, communicated, and executed throughout the organization, rests with senior management.
✔ Management should be highly responsible for implementing the pharmaceutical quality system by designing, implementing, and monitoring products throughout its product manufacturing cycle along with continuous support and communication with the entire organization.
✔ Management also defines the role and responsibility of all the organization units regarding the pharmaceutical quality system.
✔ Conduct management assessments of the pharmaceutical quality system, process performance, and product quality.
✔ Put the right resources into it.
⦿ Quality Policy: A quality policy should be established by senior management and outline the organization’s goals and plans for quality generally. The pharmaceutical quality system should be continuously improved and compliance with applicable regulatory standards should be expected under the quality policy.
✔ All employees inside the organization should be aware of and understand the quality policy. For continued efficacy, the quality policy should be reviewed on a regular basis.
⦿ Quality Planning: It clearly states that to achieve quality there shall be a policy and it shall be implemented by all levels.
✔ Management should provide appropriate training to all to achieve product quality. Performance indicators that track advancements in relation to quality goals should be created, followed, and routinely communicated.
⦿ Management of Resources: To develop and maintain the pharmaceutical quality system and continuously increase its efficacy, management needs to determine what resources (human, financial, material, facilities, and equipment) are necessary and offer them in an adequate and appropriate manner.
✔ Management should make sure that resources are used properly for a particular product, procedure, or location.
⦿ Communication: Management ensures the internal communication process is implemented within the organization which is required to ensure the appropriate information passes to all levels to achieve a pharmaceutical quality system (ICH Q10).
⦿ Periodic Review: To ensure the continued acceptability and efficacy of the pharmaceutical quality system, senior management should be responsible for its management.
✔ Management should evaluate the findings of periodic reviews of the pharmaceutical quality system, process performance, and product quality.
⦿ Outsourced Purchased Material: Management is fully responsible for the quality of outsourced purchased materials. Management ensures the proper process is followed during the supply chains, like vendor audits, material evaluation, and vendor qualification.
✔ Monitoring and assessing the contract acceptor’s performance or the quality of the provider’s materials, as well as identifying and putting any necessary modifications into place.
✔ keeping an eye on incoming ingredients and supplies to make sure they come from authorized sources while utilizing the established supply chain.
⦿ On Product Acquisition: This is a complex stage for management during the acquisition, they must ensure:
✔Each company’s ongoing responsibilities are specified.
✔The crucial information is transferred
2. Process Performance and Product Quality are Constantly Being Improved
This section describes four specific pharma quality system elements that extend the regional requirements to achieve the ich guidelines for QMS.
A. Lifecycle stage goal :
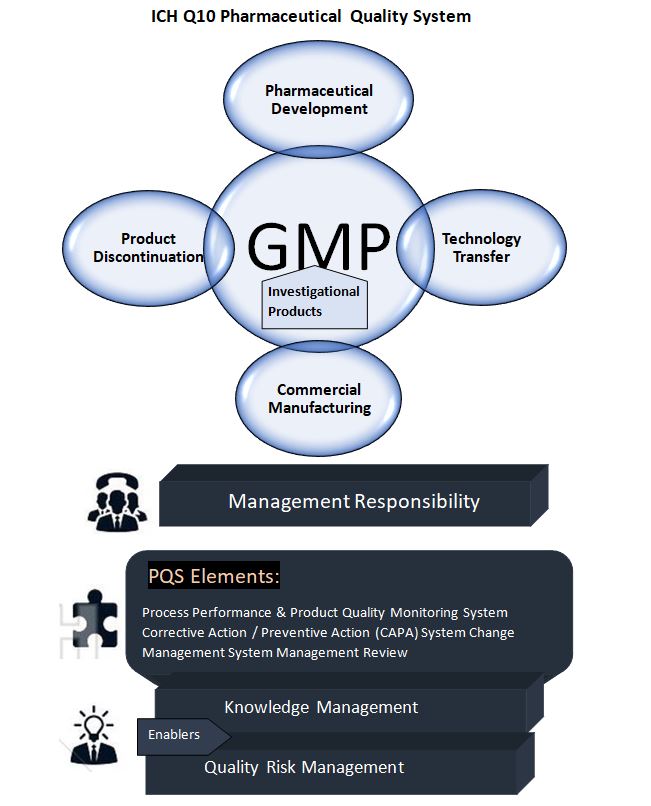
a) Pharmaceutical development: The purpose of pharmaceutical development activities is to create a product and its production process to consistently achieve the expected performance and fulfill the standards of regulatory and internal customers. ICH Q8 describes approaches to pharmaceutical development. While beyond the scope of this advice, the outcomes of exploratory and clinical development studies provide inputs to pharmaceutical development.
b) Technology transfer: Technology transfer efforts aim to transfer product and process knowledge between development and manufacturing, as well as within and across manufacturing locations, in order to ensure the quality of product realization. This concept serves as the basis for the manufacturing process, control strategy, process validation approach, and continuous improvement.
c) Commercial manufacturing: Manufacturing operations’ aims include product realization, creating and maintaining control, and allowing continuous improvement. The pharmaceutical quality system should ensure that the target product quality is regularly achieved, that adequate process performance is accomplished, that the set of controls is appropriate, that improvement possibilities are identified and assessed, and that the body of knowledge is constantly extended.
d) Product Discontinuation: The purpose of product discontinuation operations is to efficiently manage the terminal stage of the product lifecycle. For product discontinuance, a specified strategy should be utilized to handle activities such as documentation and sample retention, ongoing product evaluation (e.g., complaint management and stability), and regulatory requirements.
B. Elements of the Quality system:
These elements might be part of regional GMP rules. The Q10 model, on the other hand, aims to improve these aspects in order to promote the lifecycle approach to product quality. The four elements are as follows:
a) Process execution and product quality monitoring method: To maintain a state of control, the pharmaceutical organization should establish and implement a system for monitoring process performance and product quality. To create the control plan, the process performance and product quality monitoring system should use quality risk management. This can comprise drug substance and drug product material and component specifications and attributes, facility and equipment operating conditions, in-process controls, completed product requirements, and the associated methods and frequency of monitoring and control. The control approach should provide quick feedback/feedforward as well as appropriate corrective and preventative action.
✔Provides tools to measure parameters
✔Identify the source of variations to control variation.
b) (CAPA) Corrective action and preventive action method: A system for implementing corrective and preventive actions resulting from the investigation of complaints, product rejections, nonconformances, recalls, deviations, audits, regulatory inspections and findings, and trends from process performance and product quality monitoring should be in place at the pharmaceutical company. the investigative process should take a structured approach With the goal of discovering the fundamental cause. According to ICH Q9, the degree of effort, formality, and documentation of the investigation should be proportionate to the level of risk. CAPA approach should result in improved product and process understanding as well as improved product and process improvements.
d) Management review of process performance and product quality
The outcomes of regulatory inspections and conclusions, audits and other evaluations, and commitments to regulatory bodies should be included in the management review system.
Periodic quality assessments, which may include:
✔Customer satisfaction indicators such as product quality complaints and recalls
✔Process performance and product quality monitoring conclusions
✔Process performance and product quality monitoring conclusions
✔Any steps taken in response to past management reviews
The management review system should identify appropriate actions, such as:
✔Enhancements to manufacturing processes and products
✔Resource provision, training, and/or realignment
✔knowledge capture and distribution.
3. Continuous improvement of the PQS
A. Pharmaceutical Quality System (Management Review):
Management should have a structured framework in place for performing periodic reviews of the pharmaceutical quality system. The evaluation should include:
a) Measuring the accomplishment of pharmaceutical quality system objectives
b) Evaluation of performance indicators that may be used to assess the efficacy of pharmaceutical quality system procedures, such as:
(1) Processes for complaint, deviation, CAPA, and change management
(2) Comments on outsourced activities
(3) Self-evaluation methods, including risk assessments, trends, and audits
(4) External evaluations, such as regulatory inspections and results, as well as customer audits
B. Internal and external factors that might have an impact are being monitored in the Pharmaceutical Quality System:
Management may monitor the following factors:
a) Emerging rules, guidelines, and quality problems that may affect the Pharmaceutical Quality System
b) Innovations that may improve the Pharmaceutical Quality System
c) Changes in the business environment and goals
C. Management Review Outcomes and Monitoring:
The outcome of the management review of the pharmaceutical quality system and monitoring of
internal and external factors can include:
(a) Enhancements to the pharmaceutical quality system and related processes
(b) Allocation or reallocation of resources and/or personnel training
(c) Revisions to quality policy and quality objectives
(d) Documentation and timely and effective communication of the results of the management review and actions, including escalation of appropriate issues to senior management
Reference:
Guidance for industries Q10 Pharmaceutical quality system-FDA

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
