Quality Assurance person shall ensure the following Checklist For BMR Release. The header section of the checklist must contain the Product brand name, Product Generic name, Batch Number, Batch size, BMR number, and QA release number.
Checklist for BMR Release
- All the materials taken for production is approved?
- All the quality raw materials used for Blending/Sieving/Milling are weighed and recorded correctly.
- All the materials have been taken as per the standard quantity.
- Is the Quantity of active ingredients taken for the batch calculated correctly?
- Line clearance was taken before dispensing and counter checked by IPQA properly.
- Temperature is within the limit in the dispensing room.
- Are correct sieves used for sieving the materials as per the batch record?
- The Blending duration and blending quantities are recorded as per the batch record.
- All signatures and dates are filled properly in all columns.
- Does the compression/filling start after the approval of the blend by the QC.
- The blend report is approved and attached in BMR.
- Line clearance is taken before compression/filling.
- Compression/filling parameters are set as per the limits specified in BMR.
- Are the in-process checks performed at the frequency and recorded properly, and counter-checked by IPQA?
- In-process parameters are within limits.
- Temperature and Humidity in the compression/filling area are as per the limits.
- The QC report after compression/filling is attached to BMR.
- Is the coating started after the QC approval of compression?
- Machine setting parameters for coating are as per the limits specified in BMR.
- Are the visual checking parameters recorded properly?
- Overall signatures are filled correctly in the specified columns
- The yield at all stages is within the limits specified in BMR.
- QC report after the coating is approved and attached to BMR.
- In case of any discrepancy in the above points, fill BPRR/BPAR deviations /deficiencies form and verify the corrections made.
Note: The Footer section for the Checklist for BMR must be checked by and approved by with a sign and current date.
Related: Line Clearance Checklist point production/packing
QA Checklist for BPR

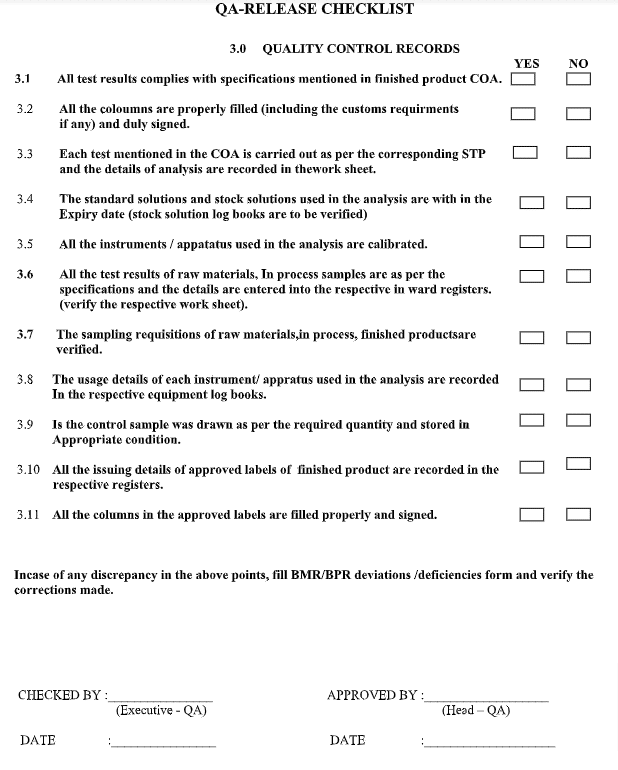
QA for Quality Control
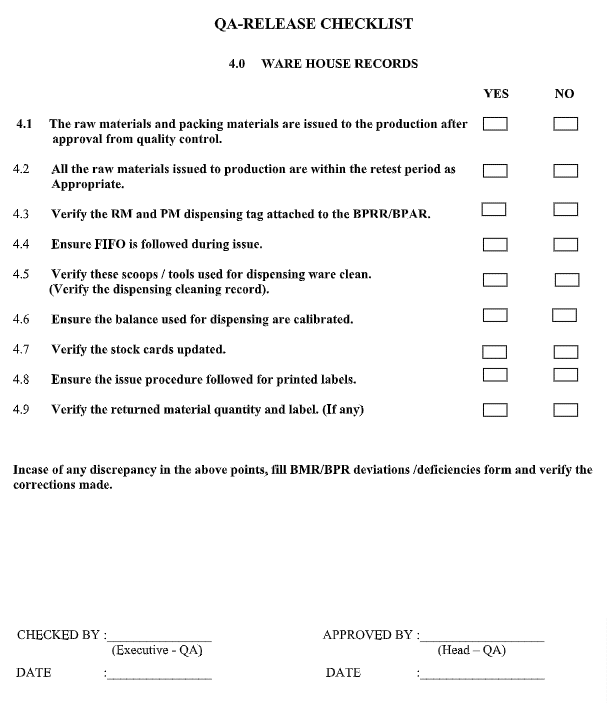
QA Checklist for Warehouse
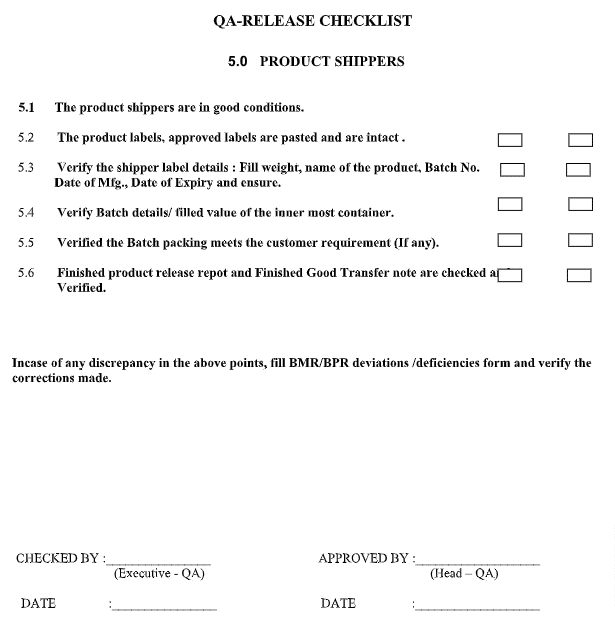
QA Checklist for Product Shipper

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
