Learn about Standard Operating Procedures on Cleaning and Sanitization in Pharmaceutical including, Aim, Scope, Responsibility, Precautions, Procedure for Different area, Frequency and Annexures.
1.0 Aim: To lay down the SOP for cleaning and sanitization of manufacturing and primary packing area.
2.0 Scope: This SOP is applicable to the entire area in the tablet department at the plant.

3.0 Responsibility officer:
3.1 Production is responsible for the implementation of this sop.
3.2 Head Production is responsible for compliance with this sop.
4.0 Precautions:
4.1 Avoid standing to pull water. Keep the area dry.
4.2 The area having no activity must be cleaned once every working day as per respective frequency and procedure.
5.0 Procedure for cleaning and sanitization:
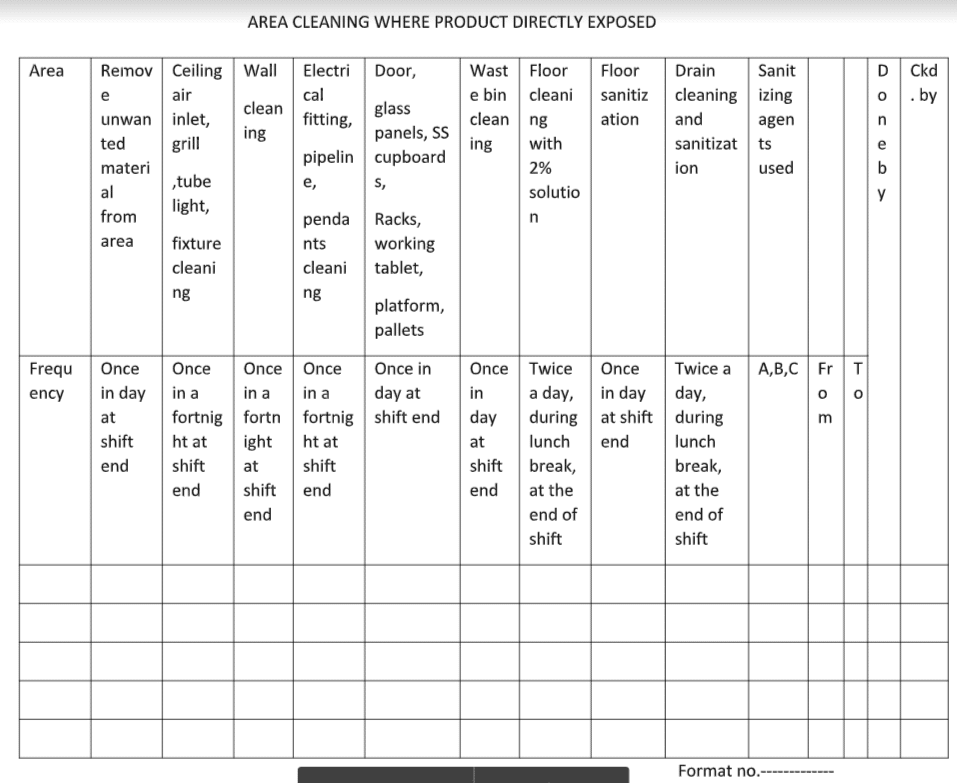
5.1 Cleaning and sensitization of area where the product is directly exposed to the environment:
5.1.1 Remove unwanted material:
5.1.1.1 Frequency:
- Once a day at shift end
- Product changeover
- Batch changes over
- Serial cleaning
- After maintenance activity
- Recleaning
5.1.1.2 Ensure that accessories or empty drums, or empty containers used are transferred to the washing area.
5.1.1.3 Ensure that the previous Batch document BMR / BPR is removed from the area.
5.1.1.4 Ensure that the previous batch material is removed from the area.
5.1.2 Ceiling, air inlet, grills, tube light fixtures cleaning
5.1.2.1 Frequency: Product changeover
5.1.2.2 Use a ladder to clean the ceiling, air inlet grill, and tube light fixture dry.
5.1.2.3 Clean the ceiling, air inlet grill, and tube light fixture by using a mop with a lint-free dryer Duster.
Related: SOP on cleaning of new equipment/machinery
5.1.3 Wall cleaning:
5.1.3.1 Frequency: Product changeover
5.1.3.2 Clean the wall using a ladder and mop with a dry lint-free Duster.
5.1.4 Electrical fitting pipelines, pendant cleaning :
5.1.4.1 Frequency: Product changeover
5.1.4.2 Clean the electrical fitting with a dry lint-free Duster.
5.1.4.3 Wipe pipelines and pendant using a ladder with a lint-free Duster (use a mop with a dryer lint-free Duster if required).
5.1.5 Doors, glass panels SS Cupboards racks working table, platform, pallet cleaning :
5.1.5.1 Frequency:
- Once a day at shift end
- Product changeover
5.1.5.2 Clean those glasses and Panels using Colin solution and dry lint-free dry Duster.
5.1.5.3 Clean the SS Cupboards, rack, working table, platform, and pallets by using a dry lint-free duster.
5.1.6 Waste Bin Cleaning:
5.1.6.1 Frequency:
- Once a day at shift end
- product change over
- Batch changes over
- After maintenance activity
- Recleaning
5.1.6.2 Remove poly bags containing the waste, for example( poly bags, paper, cable ties, etc.) from the waste Bin and clean the waste Bin with a dry lint-free Duster.
5.1.6.3 Close the poly bag and transfer it to the scrap area with an appropriate label.
5.1.6.4 Put the fresh poly bag in the waste Bin for collection of waste.
5.1.7 Floor cleaning with 2% soap solution :
5.1.7.1 Frequency:
- Once in a shift
- Product changeover
- Batch changeover
- After maintenance activity
- Recleaning
5.1.7.2 Dedust the floor using a dry lint-free Duster.
5.1.7.3 Clean the floor covering below the machine Foundation, Tables, Cupboards, etc., using a lint-free Duster socked in 2% v/v liquid soap solution.
5.1.8 Floor sanitization:
5.1.8.1 Frequency:
- On a day at Shift end
- During product changeover
5.1.8.2 Sanitize the floor covering below the machine Foundation, tables cupboard, etc., using a lint-free Duster shocked in a scheduled disinfectant solution.
5.1.9 Drain cleaning and sanitization:
5.1.9.1 Frequency:
- At the end of the Shift
- Product changeover
Note- Sanitation will only be done if there is no activity in the area.
Related SOP: Isopropyl alcohol 70 percent Preparation SOP
5.1.9.2 Remove the SS plate and sieve over the drain and “U” trap.
5.1.9.3 Clean SS plate, “U” trap using lint-free Duster soaked in 2% v/v liquid soap solution.
5.1.9.4 Rinse with soft water.
5.1.9.5 Flush the drain with about 20 liters of soft water.
5.1.9.6 Pure 2.0 liter of the disinfectant solution in the gully trap.
5.1.9.7 Use the disinfectant solution on an alternate weekly basis to prepare as per the standard operating procedure for the preparation of the disinfectant solution.
5.1.9.8 Assemble the sieve and “U” trap.
5.1.9.9 Cover the drain with the SS plate.
5.1.9.10 Record the cleaning detail as per annexure “cleaning and sanitization record of area where product directly exposed to the environment.”
5.2 Clean and sanitization the area where the product does not directly exposed to the environment:
5.2.1 Remove unwanted material.
5.2.1.1 Frequency: Once a day at Shift end.
5.2.1.2 Remove the unwanted material.
5.2.2 Ceiling, air inlet grill, tube light fixtures cleaning:
5.2.2.1 Frequency: Once a fortnight at shift end.
5.2.2.2 Clean ceiling, air inlet grille, and tube light fixture once.
5.2.3 Wall cleaning:
5.2.3.1 Frequency: Once a day at shift end.
5.2.3.2 Clean the wall using a ladder and mop with a dry lint-free Duster.
5.2.4 Electrical fitting, pipelines, and pendants cleaning:
5.2.4.1 Frequency: Once a fortnight at shift end
5.2.4.2 Clean the electrical fitting with a lint-free duster, pipeline, and pendant with net protector doors.
5.2.5 Glass, panels, SS cupboard, racks, working table, platform pallet cleaning :
5.2.5.1 Frequency: Once a day at shift end
5.2.5.2 Clean those glasses and Panels using Colin solution and lint-free dry Duster. Clean SS Cupboard, working table, platform, and pallet using a dry lint-free Duster.
5.2.6 Waste Bin Cleaning:
5.2.6.1 Frequency:
- Once a day at the Shift end
- Clean the waste Bin as mentioned in the steps above
5.2.7 Floor cleaning: with 2% soap solution:
5.2.7.1 Frequency:
- Twice a day during a lunch break.
- At the end of the Shift.
5.2.7.2 Clean the floor using a dry lint-free duster, clean the floor covering below the S.S. pallet, table, cupboard, etc., using a lint-free Duster deep in 2% V/V liquid soap solution.
5.2.8 Floor sanitization:
5.2.8.1 Frequency: Once a day at Shift end.
5.2.8.2 Sanitize the floor covering below as pallet, table, cupboard, etc., using lint-free Duster soft in a disinfectant solution.
5.2.9 Drain cleaning and sanitization:
5.2.9.1 Frequency:
- During lunch break
- At the end of the Shift
5.2.9.2 To clean and sanitize the drain
5.2.9.3 Remove the SS plate and sieve over the drain and the “U” trap.
5.2.9.4 Clean SS plate, “U” trap using lint-free Duster soaked in 2% v/v liquid soap solution.
5.2.9.5 Rinse with soft water.
5.2.9.6 Flush the drain with about 20 liters of soft water.
5.2.9.7 Pure 2.0 liter of the disinfectant solution in a gully trap.
5.2.9.8 Use the disinfectant solution on an alternate weekly basis to prepare as per the standard operating procedure for the preparation of the disinfectant solution.
5.2.9.9 Assemble the sieve and “U” trap.
5.2.9.10 Cover the drain with the SS plate.
5.2.9.11 Record the cleaning detail as per annexure cleaning and sanitization record of the area where the product is not directly exposed to environmental cleaning and sanitization of the area where the product is directly exposed to the environment.
5.3 Calibration of equipment and instrument by external party:
5.3.1 After external party calibration, cleaning is to be performed as per a schedule of running and is to be updated in a Record, “cleaning, and sanitization of manufacturing and primary packing area.”
5.3.2 Remarks column is to be done an update with appropriate comment cleaning and sanitization of the area where the product is not directly exposed to the environment after calibration of equipment and instrument by an external party.
5.3.3 After external party calibration, cleaning is to be performed as per schedule, (once a day at shift end) and is to be updated in another cleaning and sanitization of manufacturing and primary packing area remark in the column is to be updated with stable comment.
Related SOPs: SOP on Cleaning and Sanitizing Solution Preparation
Annexure-I

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at Contact@pharmaguddu.com.

very informative