Track and trace system in pharmaceuticals is a process of determining the current and past history of products through their unique ID, or their properties. It helps the manufacturer to get information about false or copied products in the market.
Track system is simply to know about the current and past location of any serialized products.
Trace system is used to know the history of who comes into contact with the products along the supply chain.
Detailed Equipment information
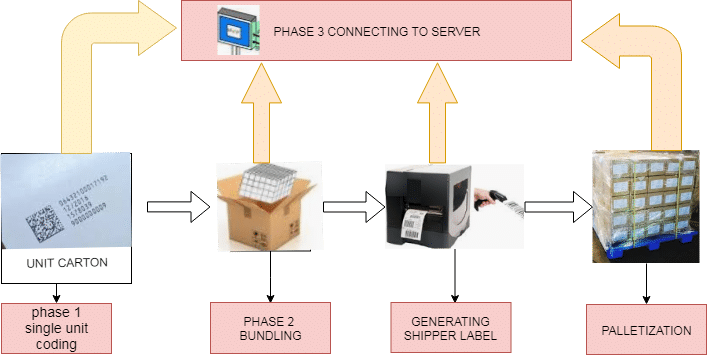
Track and Trace system installed at the secondary packaging area where the bottle or carton is to be printed, aggregated, serialized, and palatalized.
Print Area: The print Area is the area where a bottle/ carton is printed along with B.no., Mfg. Date, Expiry date, serial number, and 2D code and forward the Printed products on a conveyor belt.
360° Inspection system: 360° inspection system consists of multiple cameras around the conveyor belt to check serial no., GTIN, and 2D code on Printed products. It will automatically reject the defective products in the Rejection bin and passes the good products on the conveyor belt.
Server: The server stores the Inspection product’s information in real-time when the product is passed through the track and trace system.
Aggregation: Aggregation is the process of making a bundle. The bundle holding cartons/Bottles is again scanned manually through a barcode scanner.
Labeling: The machine automatically generates the label and fixes the label manually and automatically on the bundle.
Disaggregation: If any products are found defective during manual scanning, Later, same carton or bottle is replaced with another good one.
Related: Packing Questions for the Interview
Equipment used in track and trace system
- Printing machine (2D)
- Labeling machine
- 360° Cameras
- Bundler system
Understanding the concepts of serialization and track and trace system
Serialization is assigning a predetermined coding to each product item, assigning it with a distinct identity to be tracked and traced for its location in the supply chain or where it had been during its life cycle.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at Contact@pharmaguddu.com.
