Objective: The purpose of this experiment is to standardize 1.0 M Sulfuric Acid.

Materials used for 1.0 M Sulfuric Acid:
Equipment: Beaker, Funnel, Pipette, Burette
Chemicals: H2SO4, Phenolphthalein solution, and Anhydrous sodium carbonate.
Theory:
Methyl orange is commonly used as an indicator in titrations. In an alkaline solution, methyl orange appears yellow and has the following structure:
Procedure:
(A) Preparation of Sulfuric Acid (1.0 M):
Slowly add 30 ml of sulfuric acid to about 1000 ml of water while stirring.
Allow the solution to cool at 25°C.
(B) Standardization of Sulfuric Acid (1.0 M):
- Accurately weigh about 1.5 g of anhydrous sodium carbonate powder on a watch glass.
- Transfer the solid completely into a 250 ml beaker containing approximately 50 cm3 of distilled water.
- Wash the watch glass thoroughly using a washing bottle. Transfer all the washings into the beaker, then add more H2O to dissolve the remaining solid.
- Stir the solution with a glass rod to facilitate the dissolving process.
- Now, Transfer the solution with care into a 250 ml measuring flask using a funnel and a glass stick.
- Rinse the beaker, glass rod, and inner surface of the funnel with water, and transfer all washings to the volumetric flask.
- Repeat the rinsing process 2 or 3 times.
- Add water to add the solution volume up to 250 ml in the volumetric flask.
- Take 25 ml of the sodium carbonate solution and carefully pour it into a cone-shaped flask. Next, add a few drops of methyl orange indicator into the same flask.
- Titrate the sodium carbonate solution against the sulfuric acid solution until the color changes from yellow to reddish-orange.
Reaction:
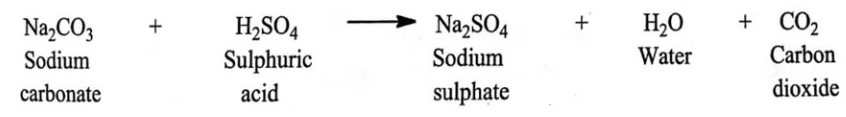
Calculations:
(A) Factor Calculations:
The molecular weight of Sodium Carbonate (Na2CO3) = 105.98 g.
From the reaction:
1 mol of H2SO4 is equivalent to 1 mol of Na2CO3.
Therefore, 1000 ml of 1.0 M H2SO4 equals to 105.98 g of Na2CO3.
Hence, 1 ml of 0.5 Molar H2SO4 (Sulfuric Acid) is equivalent to 0.05299 g of Na2CO3.
Related Post: 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
Preparation and Standardization of 1.0 M Hydrochloric Acid
(B) Determination of Normality:
1 ml of 0.5 M H2SO4 is equivalent to 0.05299 g of Na2CO3.
So, (B.R) ml of 1.0 M H2SO4 is equivalent to 1.5 g of Na2CO3.
Hence, A = 1 × 1.5 / (B.R) × 0.05299.
Result:
The normality of the given H2SO4 is found to be “A” N.

