A capsule is a type of Unit dosage form in the pharmaceutical industry. It consists of small, cylindrical components made of either soft or hard gelatin, filled with a powder or liquid. Capsules are taken orally and are designed to release their contents after swallowing. They are commonly used for medications intended to be absorbed in the stomach or small intestine. Capsules have a body, and the upper part is called the cap, with the main medicament and excipients filled in between them.
What is a Capsule?
The capsule represents a solid unit dosage form. In the manufacturing process, the drug and excipients become enclosed within a tough or soft gelatin shell. Pharmaceutical capsules serve as a container drug delivery system, offering a tasteless and odorless dosage form without the need for an additional coating step required in tablets.
Types of Capsule?
Capsules are basically categorized based on the quantity of gelatin present and come in two types: Hard Gelatin Capsules and Soft Gelatin Capsules. The basic difference between the two types of capsules is as follows:
A. Hard Gelatin Capsules
- They contain fewer plasticizers compared to SGC.
- HGC consists of two parts: a Cap and a Body.
- They are primarily used for filling powders, granules, and pellets.
- Manufactured through the dipping method, involving steps like Dipping, Rotation, Drying, Stripping, Trimming, and joining. However, nowadays, the process has been automated, making it a two-step process where the empty capsule shells are first manufactured separately and then filled using various equipment.
Formulation of Hard Gelatin Capsules:
A. Gelatin: Gelatin is prepared by hydrolyzing collagen obtained from animal connective tissue and bone skin. Upon hydrolysis, low polypeptide chains yield 18 amino acids, with glycine and alanine being the most prevalent. Gelatin’s physical and chemical properties can vary depending on the collagen source and extraction method. There are two basic types of gelatin.
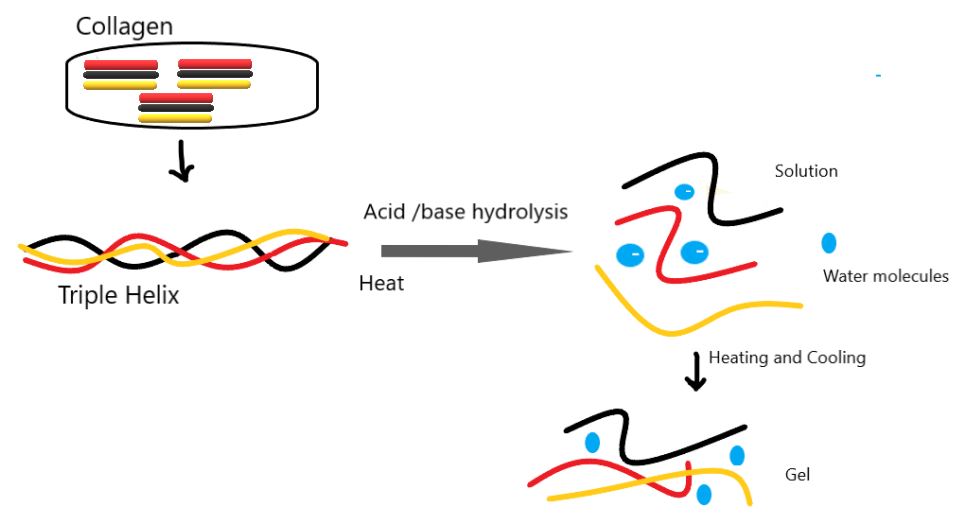
Type a. (Acid-hydrolyzed gelatin), formed through acid hydrolysis, mainly comes from pork skin.
Type b. Alkaline hydrolysis forms (base hydrolyzed gelatin), which is mainly produced from bones. Differentiation between the two types can be done based on their isoelectric point. Gelatin A type has an isoelectric point near pH value 9, while Type gelatin B has an isoelectric point near pH value 4.7.
B. Opacifying agents used in HGC include titanium dioxide. Opaque capsules serve to provide protection from light or to cover the contents.
C. Preservatives: The most common preservatives used in capsules are Methylparaben (lipid-soluble) and propyl Parabens (aqueous-soluble).
D. lubricants: Lubrication is used to lubricate the granules or powder-filled in capsules quantity less than 2%.
E. Moisture content: The finished hard gelatin capsule typically contains a stable moisture content of 12 to 15%, determined by the toluene distillation method. This moisture content is crucial for the physical properties of the shell. A lower moisture content causes the gelatin film to become brittle, while a moisture content of less than 18% leads to softness in capsules. Capsules are stored at a relative humidity of 40-60% and a temperature of 21°C – 24°C to prevent extreme temperature exposure during the handling and sorting processes.
Excipients used in Capsules:
Types of Excipients used in powder-filled capsules as follows:
Diluents— Diluents are the excipients that generally have the highest concentration in a formulation, and they provide the necessary bulk when the active ingredient quantity is insufficient to meet the required volume, for example, lactose, maize starch, calcium sulfate, etc.
Lubricants and Glidants— This reduces powder-to-metal adherence and enhances free-flow properties, for instance, magnesium stearate and talc.
Wetting agents— A wetting agent aids in the penetration of water for less soluble drugs, such as sodium lauryl sulfate.
Disintegrates— Disintegrates assist in breaking down the powder mass, for example, crospovidone and sodium starch glycolate.
Hard gelatin capsules filling Process:
Hard gelatin capsule filling is done by the following process:
Rectification: During Rectification empty capsules are oriented so that they can be laying in the same direction, i.e., laying the capsule Body end in a downward direction. Moving forwards caps will separate from the Body as the upper plate slides towards the left, leaving the lower plate exposed to the filling. it leads to feeding coming from the hopper filling the capsule body. the last step is the replacement of caps on the Body and the ejection of filled capsules from the die, parallelly de-dusting, polishing, and printing is done.
Equipment used for Filling Hard Gelatin Capsules:
1. Lilly Park Device
2. Rotofil
3. Hofliger
4. Macofar
5. MG2
6. Osaka
7. Zanasi
8. Perry/Accofil.
In the case of Farmatic, Rotofil, Macofar, MG2, and Zanasi equipment, the power must be sufficient to maintain cohesiveness to retain the slug or pellet during delivery to the capsules.
Equipment used for De-dusting Hard Gelatin Capsules:
1. Rotosort: A mechanical sorting device that removes loose powder and unfilled joined capsules, whether filled or unfilled bodies and loose caps.
2. Erweka KEA: Used for both de-dusting and polishing.
3. Seidender Machine: Utilized for visual inspection using a belt.
Rotator Bottle Method: is used for dissolution rate studies in capsules.
Test for Gelatin includes:
- Picric acid test: A 10% solution of picric acid is used to determine the presence of gelatin.
- Xanthoprotein test: This test helps detect the presence of sulfur-containing amino acids.
- Ninhydrin Test: It identifies amines, ammonia, or primary and secondary amides.
- The tannic acid test, which gives white ppt of Gelatin because it is protein and tannins ppt the protein.
Advantages of Hard gelatin capsule:
- The Gelatin shell rapidly dissolves and ruptures, leading to the quick release of a drug.
- Capsules do not require compression force for manufacturing, unlike tablets. This results in higher bioavailability compared to tablets.
- Capsules are suitable for hygroscopic and/or moisture-sensitive drugs as they can be formulated without any excipients.
- They have a smooth surface, making them easy to swallow.
Disadvantages of Hard gelatin capsule:
- The Gelatin shell is prone to microbial growth, thus requiring preservatives to increase the shelf life of capsules.
- Highly soluble salts such as iodides, bromides, and chlorides should generally not be dispensed in HGC.
- They may cause gastric irritation due to the rapid release and formation of high drug concentrations in localized areas.
- Gelatin is an animal-derived ingredient, making it unsuitable for vegetarians and vegans.
- Hard gelatin capsules are not suitable for moisture-sensitive drugs as they have a high moisture content.
- They have limited flexibility in terms of size and shape compared to other types of capsules.
The solubility limits of empty capsules are:
(a) Water resistance: Fails to dissolve in 1-10 at 20-30°C in 15 Minutes.
(b) Acid solubility: Dissolves in less than 5 minutes in 0.5% aqueous HCl at 36-38°C.
In capsule evaluation, the following terms are used:
Soft spot: The site where the capsule lies next to the tray or against another capsule dries slowly.
Bloating: In this case, capsule size enlarges (bloated) if stored at high humidity (60%).
Foreign capsules: Unfilled capsules are referred to as foreign capsules.
Difficulties in filling capsules:
- Deliquescent or hygroscopic powders absorb the water present in gelatin, causing the capsule to crack. Adding an absorbent like magnesium carbonate, heavy magnesium oxide, or light magnesium oxide can overcome this issue, provided the capsules are packed in tightly closed glass vials.
- Eutectic mixtures result in a pasty mass formation due to the liquefaction of substances when mixed together, as they form a mixture with a lower melting point than room temperature. To prevent these issues, mix the substances separately before combining them and filling the capsules.
B. Soft Gelatin Capsules
Soft gelatin capsules contain a drug that is encapsulated, dissolved, solubilized, or suspended in a liquid vehicle. Lecithin, the wetting agent, is used in a suspension that must fill a soft gelatin capsule. They are sometimes known as soil gels because they are made from a more plasticized gelatin film. They are completely sealed dosage forms that cannot open without destroying the shell. Generally, formulations enclosed in the SGC are mostly liquids like suspensions or solutions of drugs that do not solubilize the gelatin shell.
The most common method for preparing SGC is:
- Rotary die process
- Plate process
- Bubble method
- Accogel (for filling powders or granules in SGC) Depending on the machine tooling, they may become spherical, oval, oblong, tube, etc.
Composition of Soft Gelatin Capsules:
A. Gelatin: it is plasticized by using the plasticizer, sorbitol, glycerin, and propylene glycol. The ratio of dry plasticizers to dry gelatin measures the hardness of the capsule shell.
The hardness of gelatin shell = Dry weight of plasticizer / Dry weight of Gelatin
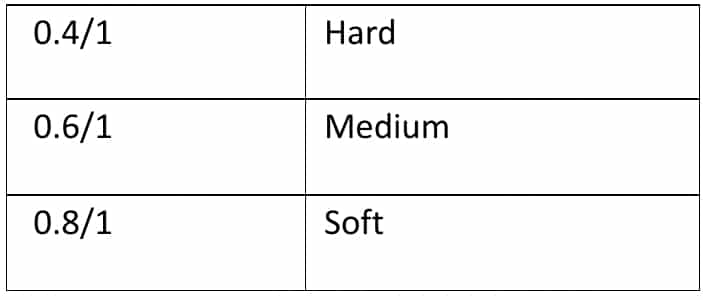
The basic gelatin formulation for casting plasticized films typically consists of one part Gelatin, one part Water, and a 0.6-0.8 part plasticizer. The moisture content within the gelatin shell falls between 6-10%.
B. Preservatives: Preservatives are generally added up to 0.2%, with the most common combination being 4 parts of methylparaben and 1 part of propylparaben. Other preservatives, such as beta-naphthol, can also be used.
C. Opacifier: Titanium dioxide is used in the concentration range of 0.2% to 1.2%.
D. Fumaric acid: To increase the solubility of acids and reduce the aldehydic tanning of gelatin, 1% fumaric acid is added.
E. 5% Sugar: Sugar is added to form a chewable shell and enhance the taste.
F. Essential oils: The concentration of essential oils is about 2% for increased taste and odor.
Suggestions and Precautions:
- Iron content should not exceed 15 PPM to prevent color spots caused by the reaction with colorants. Iron enters the gelatin during the hot extraction process.
- To prevent the decomposition of gelatin, 0.15% sulfur dioxide (SO2) should be added.
- Formaldehyde hampers the disintegration of the gelatin shell by initiating cross-linkage in the gelatin molecule.
- Other agents that alter the solubility of gelatin include Salol, shellac, and CAP.
The Physicochemical properties of Capsule gelatin
1. Bloom strength
2. Viscosity.
- Bloom strength: Bloom strength refers to the force required to compress a gelatin capsule. A capsule with high bloom strength is typically considered good quality as it can withstand handling and packaging without breaking or leaking. Lower bloom strength may indicate a weaker capsule that is more prone to breaking or leaking. The empirical gel strength measure determines the molecular weight of gelatin, giving an indication of the firmness of the gel, i.e., cohesive strength or internal cross-linking. It is measured using a Bloom Gelometer, which determines the load in grams required to depress a typical plunger (0.5 inches in diameter) a hard and fast distance of 4 mm deep into (6.66%) the raw materials gelatin with the highest bloom strength. Bloom strengths in the range of 150-280 gm are considered suitable for capsules.
- Viscosity of gelatin: The viscosity of gelatin is used to measure the chain length of gelatin. The viscosity of gelatin solutions is vital for controlling the thickness of the cast film or essential for the manufacturing characteristic of gelatin film. Viscosity is determined using 6.66% w/w of gelatin solution in water at 60°C using a capillary pipette. The range must be 25-45 millipoise.
- The sealing temperature of the gelatin film ranges between 37-40°C.
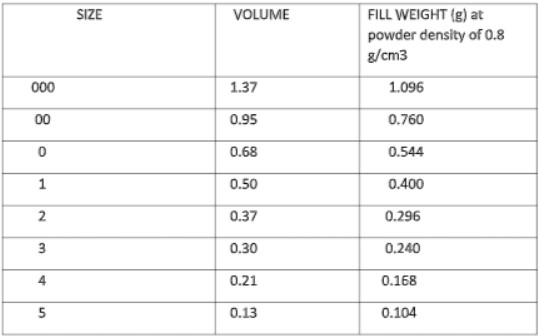
- The minimum fill volume in capsulation is calculated from the specific gravity of a liquid.
- Capsules at equilibrium with 20-30% relative humidity at 21-24°C are considered dry, and the shell of such capsules contains 6-10% water depending upon the gelatin formula used.
- The moisture content of the shell is determined by the toluene distillation method.
Advantages of Soft gelatin capsule:
- They are easy to swallow and ideal for patients who have difficulty swallowing.
- Can mask the unpleasant taste or odor of certain drugs.
- Better bioavailability compared to tablets as they do not require disintegration before dissolution in the stomach.
- Can provide sustained release of drugs by changing the formulation or properties of the gelatin shell.
Disadvantages of Soft gelatin capsule:
- There is more intimate contact between the shell and liquid contents than with dry-filled HGC, increasing the possibility of interactions. For example, chloral hydrate formulated with oily vehicles exerts a proteolytic effect on the gelatin shell; however, this effect can be reduced by replacing the vehicle with PEG.
- Drug components can drift from oily liquid vehicles to the surface of Gelatin depending on their partition coefficient because the gelatin shell has a certain percentage of moisture. For example, solutions encapsulated with 4-hydroxybenzoic acid.
- The pH of the liquid can range from 2.5 to 7.5, and a more acidic pH liquid would favor causing hydrolysis, leading to leakage. A pH greater than 7.5 and aldehydes decrease shell solubility through the tanning of the Gelatin.
- Aqueous emulsions cannot fill soft gelatin capsules because they may release water, affecting the shell.
Determination of Base absorption and M/G factor:
Base absorption defines the minimum amount of base or vehicle in grams required per gram of solid drug to form a mixture that can be easily encapsulated in SGC. It is a unitless value because both parameters are in grams.
M/G factor: When the soft gelatin shell contains a suspension, determining the M/G factor becomes crucial to find the minimum quantity of base / inert liquid/vehicle needed to form the suspension. If the concentration of solids is high, the flow of material to the injection wedge is hindered.
The formula for determining Base absorption is = Weight of base (grams) / weight of drug (1 gram). The drug is placed in a beaker, and the vehicle or wetting agent (5% lecithin) is added at a rate of 1 ml/min each time. It is triturated (at an angle of 45°C) until a uniform flow is achieved. Then, stop adding oil and measure the consumed volume. The value depends on the following factors:
- Particle size and distribution
- Density of drug
- State of the drug (crystalline, amorphous)
- Hydrophobicity and hydrophilicity of the drug.
Determination of M/G factor: The above uniform mixture obtained is homogenized in a colloid mill and subject deaeration in a desiccator and find out the specific gravity/density.
M/G factor formula =BA + S x 16.23 minims /D
BA: Base absorption value, S: Solid drug, D: Density of mixture, 1 ml = 16.23 minims
M / G factor is defined as the volume of mixture in minims required for a solid drug to produce a mixture that can be encapsulated, e.g., M / G = 50 mean I gm of the drug in a mixture occupies 50 minims. Thus lower the adsorption of the solid (s) and the higher the density of the mixture, the smaller the capsule size will be.
Quality Control Test for Capsules:
Before releasing a product to the market, it is necessary to ensure compliance with various tests such as the disintegration test using the D.T. Apparatus, weight variation test, and percentage of medicament test. Additionally, a visual inspection is conducted to verify uniformity in shape, size, color, and filling. During inspection, any capsule defects, whether visibly defective or suspected, are identified and removed. Both hard and soft gelatin capsules must undergo standardization tests.
- Shape and size
- Color
- The thickness of the capsule shell
- Leakage test
- Disintegration tests
- Weight variation test

Disintegration Test: According to B.P., perform the Disintegration test on capsules. Use a disc when necessary. Place one capsule in each tube, then suspend them in beakers. Move them up and down for 30 minutes. Unless stated otherwise in the monograph, the capsules should pass the test with no residue of the drug or fragments of shells larger than mesh size 10 remaining.
Weight Variation Test: Conduct the weight variation test for capsules using 20 capsules randomly selected. Weigh and calculate their average weight. A capsule passes the test if its weight falls within 90-110% of the average weight. If the requirements are not met, determine the weight of each capsule’s content and compare it with the average content weight.
Remove shell contents by emptying them or using a small brush. For soft gelatin capsules, press the content after careful cutting. Wash out the remaining contents with a suitable solvent. After drying the shells, weigh them, and calculate the content weights of individual capsules.
The requirements are met if no more than two differences exceed 10% of the average net content, and in no case does the difference exceed 25%.
Capsule stability: Unprotected soft capsules (i.e., capsules that breathe) quickly reach equilibrium with the atmospheric conditions in which they are stored. This inherent characteristic necessitates a discussion on the consequences of temperature and humidity on these products, highlighting the importance of appropriate storage and packaging conditions, as well as the selection of a suitable retail package. Establishing physical standards for each product is imperative due to the variety of materials encapsulated that can affect the gelatin shell and the different gelatin formulations used.
General statements concerning the effects of temperature and humidity on soft gelatin capsules should specifically focus on capsules containing oil (mineral oil) with a gelatin shell having a dry glycerin to dry gelatin ratio of approximately 0.5 and a water to dry gelatin ratio of 1:1, dried to equilibrium with 20 to 30% RH at 21 to 24°C. The physical stability of soft gelatin capsules is linked to the absorption or loss of water by the capsule shell. Adequate packaging should prevent these issues, ensuring satisfactory physical stability within a temperature range from slightly above freezing to as high as 60°C. For unprotected capsules, low humidities (less than 20% RH), low temperatures (less than 2°C), high temperatures (greater than 38°C), or combinations of these conditions only have temporary effects.
Reference: US Pharmacopeia for capsules and hard gelatin capsule
Frequently Asked Questions (FAQs):
A: M/G factor stands for Minims per Gram and is a critical measurement in capsule formulation that indicates the volume of mixture required for a solid drug to produce a mixture capable of being encapsulated. It helps in determining the appropriate capsule size and ensuring proper flow during the manufacturing process.
A: Quality can be ensured through a series of tests such as the disintegration test using a D.T. Apparatus, weight variation test, percentage of medicament test, and a thorough visual inspection for any capsule defects. These tests verify capsule uniformity, integrity, and compliance with pharmacopeial standards.
A: For the disintegration test, place one capsule in each tube of the D.T. Apparatus and submerge them in beakers filled with liquid. Move them up and down for 30 minutes. To pass, there should be no residue of the drug or fragments of shells larger than a specified mesh size remaining.
A: If a capsule’s weight does not fall within 90-110% of the average weight, examine the weight of each capsule’s content individually. Comply with the test requirements by ensuring not more than two capsules exceed 10% deviation from the average net content, and none exceed a 25% difference.
A: Temperature and humidity can significantly affect the physical stability of soft gelatin capsules by causing the shell to absorb or lose water. It’s crucial to employ appropriate storage and packaging conditions to prevent such issues and maintain physical stability under various environmental conditions.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].


Very informative article on Capsules , Thank you