Disintegration test apparatus is widely used in pharmaceuticals to identify the disintegration properties of various types of tablets and capsules. The DT apparatus decides whether or not tablets or capsules disintegrate within a recommended time once placed in a liquid medium.
What are Disintegration and Principle
Disintegration is defined as that state in which no residue of the tablet and capsule remains on the screen of the apparatus or, if a residue remains, it may consist of a fragment of insoluble coating of the tablet or capsule shells or is a soft mass with no palpable core.
If Discs have been used with capsules, any residue remaining on the lower surface of discs only consists of fragments of their shells.
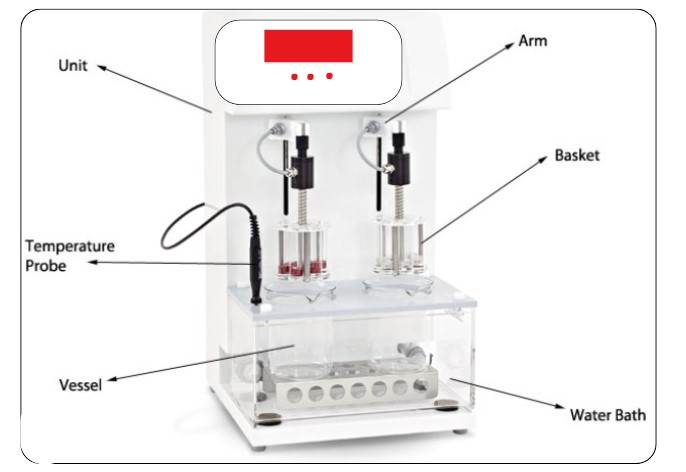
Types of Disintegration Test Apparatus
Disintegration test apparatus are mainly of two types, Type A and Type B:
Type A: It consists of 6 tube assembly mainly used for tablets of size less than 80 mm in length.
Type B: It consists of a 3-tube assembly used for tablets of size more than 80 mm in length.
Disintegration Test apparatus assembly
DT apparatus assembly Consists of the following parts:
- Rigid Baskets
- Tube
- Plates
- Cylindrical Disks
- Assembly
- Thermostat
1. Rigid Baskets:
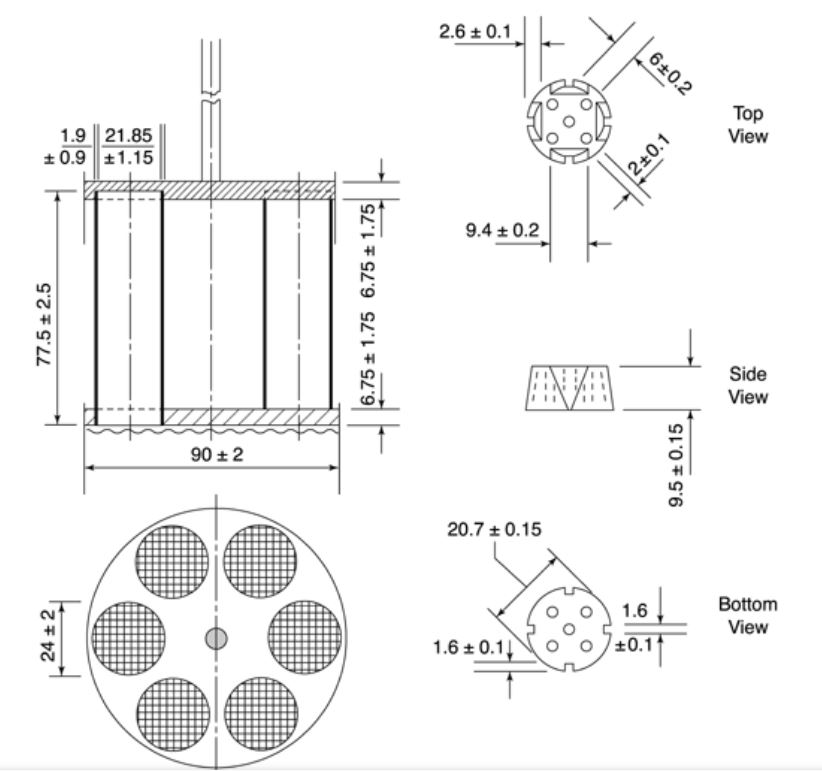
The rack assembly supports six cylindrical glass tubes, 77.5 ± 2.5 mm long, 21.5 mm in internal diameter, and a wall thickness of about 2 mm.
2. Tube:
The tube is held vertically by two superimposed, transparent plastic plates, 90 mm in diameter and 6 mm thick, perforated by six holes having the same diameters as tubes.
The hole is equidistant from the center of the plate and is equally spaced from one another, that is attached to the lower side of the lower plate is a piece of woven gauze made from stainless steel wire (10 mesh screen size). These are 635 mm in diameter and have a nominal mesh aperture of 2.00 mm.
The upper plate is secured with a tempered stainless steel circle punctured by six gaps, each around 22 mm in breadth, accommodating our cylinders and holding them between the plastic plates. The hole coincides with those of the upper plastic plate and the upper open ends of glass tubes.
3. Plates:
Plates are firmly held in place and are 77.5 mm apart by vertical metal rods on the sides. The center of the upper plate is fixed to the metal rod, allowing the assembly to be connected to a mechanical device capable of quickly raising and lowering it at a constant frequency of between 29 and 32 cycles per minute over a distance of 50 to 60 mm.
The design of the basket-rack assembly may be somewhat different provided specifications for the glass tubes, and the screen mesh sizes are unchanged.
Related: pH Meter | Principle, Calibration, and Working
4. Cylindrical Disks:
The holes in the disk have dimensions of 20.7 ± 0.15 mm and a thickness of 9.5 ± 0.15 mm. The disk is made of transparent plastic with a density ranging from 1.18 to 1.20. It has five holes, each 2 mm in diameter. One hole is at the center, and the other four are equally spaced in a circle with a radius of 6 mm from the center of the disc. Additionally, four grooves are cut on the horizontal surface of the circle. These grooves are 9.5 mm wide and 2.55 mm deep on the upper surface of the plate, and 1.6 mm square on the lower surface.
5. Assembly:
In a suitable container, preferably a 1000 ml beaker, the assembly is placed in the liquid. The wire mesh should be at least 25 millimeters below the liquid surface, and its lower end should be at least 25 millimeters above the beaker.
6. Thermostat:
A Thermostat is an Arrangement for heating the liquid and maintaining the temperature around 37 ± 2° C.
Disintegration Test Procedure
Thinking about how to perform the Disintegration test? Let under their methods and different stages:
Unless otherwise explicit within the individual monograph, introduce one tablet or capsule into every glass tube or six tablets. If directed within the acceptable general monograph, add a disc to every tube.
Suspend the assembly within the beaker containing the desired liquid, operate the equipment for the desired time, and take away the assembly from the liquid. The tablets and capsules pass the test if all of them have completely disintegrated.
L1, L21 and L3 stage to perform:
| Stage | Stage+No. of Tablets | Pass/ Fail Criteria |
|---|---|---|
| L1 | L1 —6 | All Tablets/ Capsules shall be Disintegrates |
| L2+L3 | L1+L2+ L3 — 18 | Not less than 16, of a total of 18 tablets/capsules |
If one or two tablets/capsules fail to disintegrate, repeat the test on 12 additional tablets/capsules; not less than 16, of a total of 18 tablets/capsules, tested for disintegration.
If the tablets or capsules adhere to the disc and the examined preparation fails to comply, repeat the test omitting the disc. The preparation complies with the test if all the tablets or capsules disintegrate in a repeat test.
Calibration of Disintegration Test Apparatus
- Start the machine and count how many times it operates in a minute.
- Check the distance traveled by the mechanical device holding the cells and discs. Use vernier calipers to measure the distance. Make sure the device moves smoothly covering a distance of 50 to 60 mm consistently.
- Record the number of cycles per minute using a calibrated stopwatch.
- Set the required temperature, turn on the heater of the machine, and maintain a stable temperature.
- Examine the water bath temperature with a calibrated thermometer.
- Set the timer for 30 minutes, and start the machine and the calibrated stopwatch together. Note the stopwatch reading as soon as the machine stops.
- Visually inspect the integrity of the sieve attached to the baskets.
- Attach a calibration status label, filled and signed, on the equipment after completing the calibration.
- If you notice any issues, inform the Head of the department and the engineering department about the necessary actions. Place an ‘UNDER MAINTENANCE’ tag on the machine.
- Recalibrate the machine after fixing any issues.
Frequency: once a month.
Related: Various Types of HPLC Columns
Different types of tablets Disintegration times
| Tablets Types | Disintegration Time |
| Uncoated tablets | 15 min (BP, IP), 30 min (USP) |
| Film-coated | 30 min (BP), others coated tablets 60 min |
| Sugar-coated | 60 min (BP) |
| Effervescent tablets | <5 min (BP, IP) |
| Dispersible tablets | <3 min (BP, IP) |
| Enteric-coated tablets | With 0.1N Hcl- No disintegration in 120 min With 6.8 pH phosphate buffer in 60 min |
| Hard gelatin Capsules | 30 min as per BP, and USP |
| Soft gelatin Capsules | 30 min as per BP |
Related post: List of Quality Control Equipment in Pharmaceuticals
FAQs
Ans: State at which no residues remain of tablets on-screen of apparatus, besides fragments of coated tablets.
Ans: 55 mm ± 2 strokes/minute.
Ans: Disintegration time is the time required to break the unit dosages into small granules under specified conditions.
Ans: The disk is used to Allow the tablets to stay deep in the water during the entire process.
Reference:
- Pharmacopeia general chapters <701> Disintegration

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
