Pharmaceutical Sampling involves selecting a portion of a product for a specific purpose. The sample is a part of a material collected following a defined sampling procedure. A Sampling plan specifies the location, the number of units, the quantity of material to collect, and the associated acceptance criteria. The Sampling procedure contains all sampling operations conducted on a specific material for a particular purpose. A Sampling unit is a distinct part of a consignment, like an individual package, drum, or container.
Types of Pharmaceutical Samples
Original sample: The original sample is the sample collected directly from the material.
Combined or Pooled Sample: A combined or pooled sample is a sample from all combined parts.
Final Sample: The final sample is the one ready for utilization for the test method.
Random Sample: A random sample is a sample in which the different portions of the material have an equal possibility of being characterized.
Representative Sample: A representative sample is a sample obtained consistent with a sampling procedure designed to ensure that the various parts of a batch or the various properties of a non-uniform material are proportionately represented.
Retention sample: A retention sample is collected as part of the initial sampling process and reserved for further testing in the future. The retention sample size should be sufficient to allow for at least two confirmatory analyses. In some cases, statutory regulations may have more than one sample, each of which should be separate, determined, packed, and sealed.
Purpose of Pharmaceutical Sampling
Pharmaceutical sampling serves various purposes such as prequalification, accepting consignments, releasing batches, testing in-process control, implementing special controls, inspecting for customs clearance, and checking for deterioration or adulteration.
The test applied for the Retention sample may include:
- Verifying the identity.
- Performing complete pharmacopoeial or analogous testing.
- They are performing special or specific tests.
Design of Pharmaceutical Sampling Facility
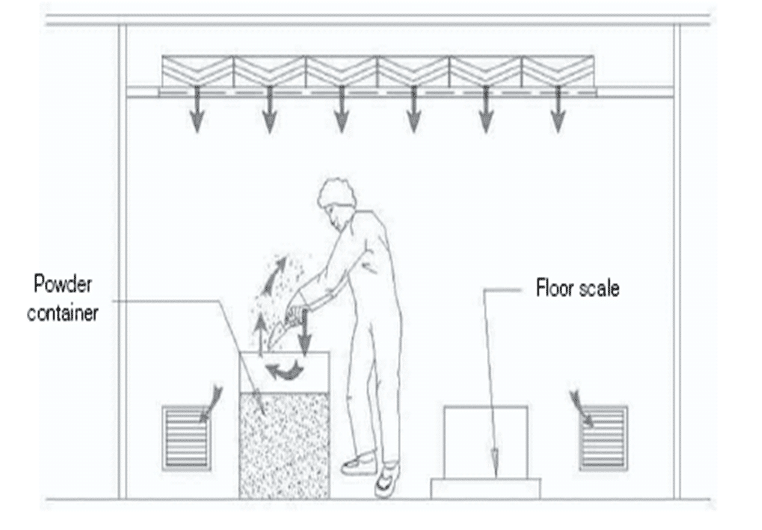
Pharmaceutical sampling facilities must be designed to prevent contamination of pharmaceutical intermediates and in-process containers and to avoid cross-contamination from other materials, products, and the environment. The sampler conducting the sampling process needs protection.
Preparation for sampling
The sampler should wear gowning for protection during tasks. In addition, the sample storage areas must have adequate lighting conditions, and ventilation care should be taken to guard against the collapse of stacked containers or bulk solids. Ensure that safety data sheets (MSDS) for pharmaceutical products and related materials are available.
Sampling Tools
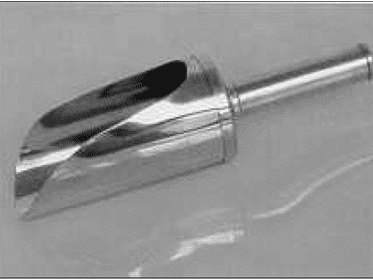
A sampling of uniform starting materials includes:
- Different types of pipettes, fitted with suction bulbs, cups, beakers, dippers, and funnels, are necessary for liquids with low viscosity.
- An inert rod can be utilized for highly viscous liquids, while spatulas or scoops are essential for powdered and granular solids.
- Sampling sterile products should occur under aseptic conditions and only when essentially permitted to avoid the risk of sterility loss.
- The use of glass should be avoided.
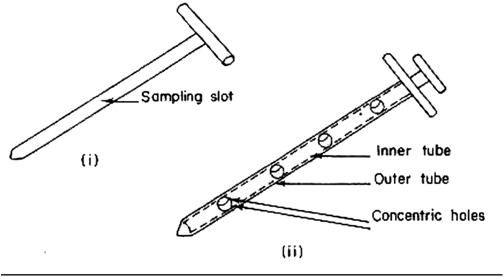
A sampling of non-uniform materials:
- A sampling tube with a shutter at the lower part can sample liquids in containers or other large-volume containers. For solids sampling, a slotted tube with a pointed part is suitable.
Note: All pharmaceutical sampling tools and implements should consist of inert materials and require careful cleaning after use or before reuse. They must undergo thorough washing, rinsing with water or a suitable solvent, and drying. Record and document all cleaning procedures online to maintain traceability.
Sampling Operation and Precautions
Written procedures that describe the Pharma sampling operation ensure the collection of representative samples in adequate quantities for testing as per specifications. Samples should not be returned to the bulk container. The sampling procedure must be followed, monitored, and any signs of material non-conformity should be carefully observed. Signs of non-uniformity include…
- Variation in particles in shape, size, or color, crystalline, granules, or powdered solid materials;
- Moist crusts on hygroscopic materials;
- Deposits of the solid product in liquid or semi-liquid products; and a layer of liquid products.
Avoid pooling samples from different portions because this practice masks contamination, low potency, or other quality issues. Ensure that labeling is easily identifiable with relevant details that include the batch number and, if available, the container number from which the sample was taken, along with the quantity taken.
If a container has been punctured for sampling, the sampling hole must be properly closed and labeled as such. Sample containers must be identified, as they may not contain the amount of product indicated on the label. The RLAF of the sampling booth should be activated at least 15 minutes before sampling begins, and only one batch should be sampled at a time.
Containers for Pharmaceutical Samples
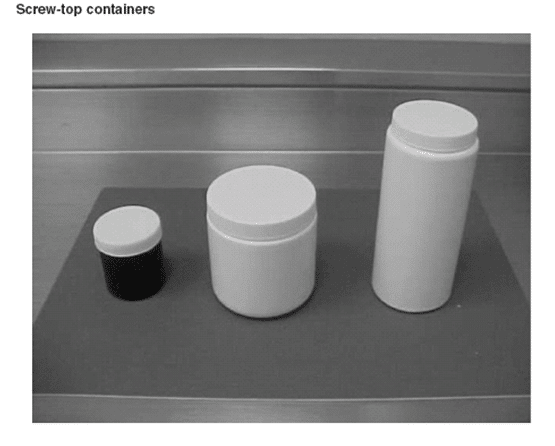
The container for storing a sample should not interact with the sampled material nor allow contamination. Bottles closed by screw tops with inert liners should be used for liquid samples that require vapor-proof protection for light-sensitive materials. These liquid samples should be protected by either amber glass containers or dark-colored foil.
Headspace must be minimized to reduce potential degradation. When transporting solid dosage forms like tablets or granules, ensure their protection by filling the container with suitable materials or filling any remaining space with an appropriate substance. Follow the provided storage conditions for sample preservation.
Types of Sampling Plans in Pharmaceutical
n Plan: Assuming a uniform material from an identified source with the primary purpose of checking the identity.
Note: “n-plan” is not statistically based and will be used only as a per guideline.
Material to be sampled is uniformly taken into account and supplied from a recognized source. Samples are often withdrawn from any part of the container, usually from the top layer, using the formula: n = 1 + √N, where N represents the number of sampling units in the consignment. The value of n is obtained by simply rounding n if N is a smaller amount or up to 4; in this case, every container is sampled. Sampling units are randomly selected and subsequently placed in separate sample containers. The control laboratory inspects the appearance of the material, tests the identity of every original sample consistent with the relevant specification.
p Plan: The material is uniform, originates from a recognized source, and serves primarily for identity testing. The formula is p = 0.4 under the square root of N, where N represents the number of sampling units. The figures for the p plan are determined by rounding up to the next highest integer. Samples are drawn from each or every N sampling unit of the consignment and stored in separate containers. These samples should then be transferred to the control laboratory for visual inspection and identity testing. If the results prove satisfactory, p final samples are created by appropriately pooling the original samples.
r Plan: The material is suspected to be non-uniform and originates from an unknown source. The formula is r = 1.5 √ N, where N represents the number of sampling units. The values for r are determined by rounding up to the next highest integer. Samples are taken from each or every N sampling unit of the consignment and stored in separate containers. These samples should be transferred to the control laboratory for identity testing. If the results are satisfactory, r samples are randomly chosen and individually tested. Upon satisfactory results, the samples are combined for the retention sample.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at Contact@pharmaguddu.com.
