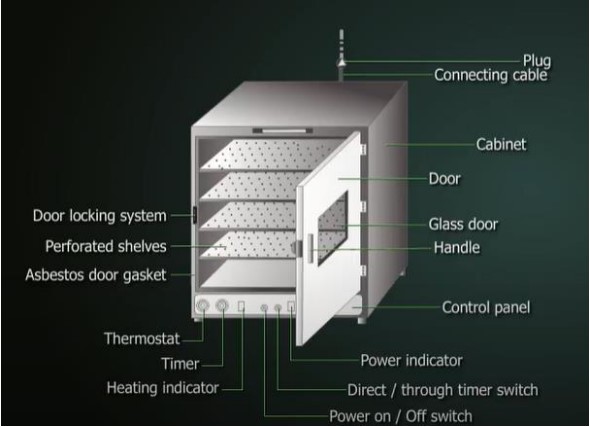
ISO 14644‑1 Overview and Recent Revision
The ISO 14644‑1 standard (2015 edition) is the global benchmark for cleanroom air cleanliness, defining nine classes (ISO Class 1–9) based on airborne particle counts. Each class sets maximum concentrations of particles ≥0.1, 0.2, 0.3, 0.5, 1.0, or 5.0 μm in a cubic meter of air. In practice, lower class numbers mean cleaner environments. For example, ISO Class 5 (used in … Read more