Top 10 Common Audit Findings in Pharmaceutical Manufacturing
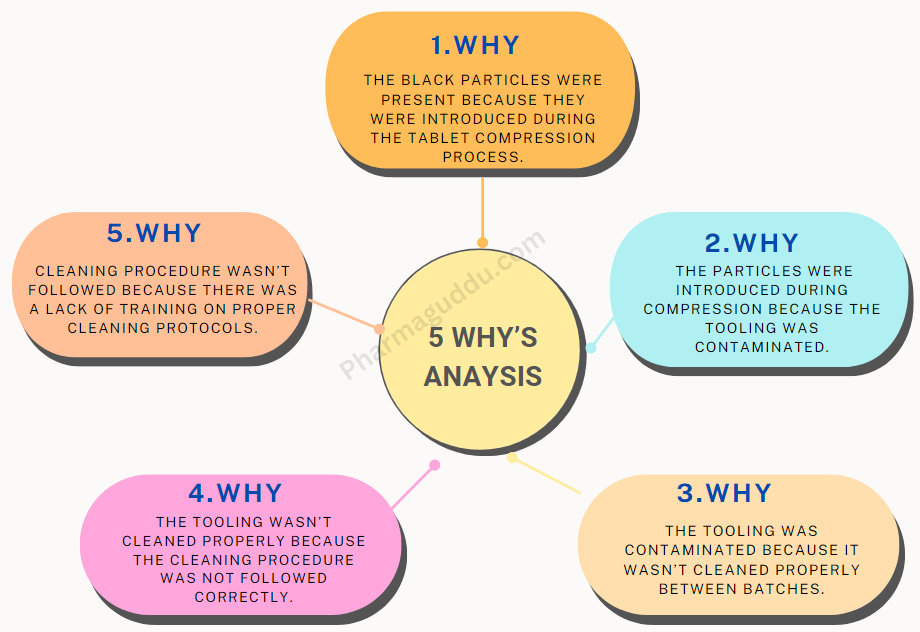
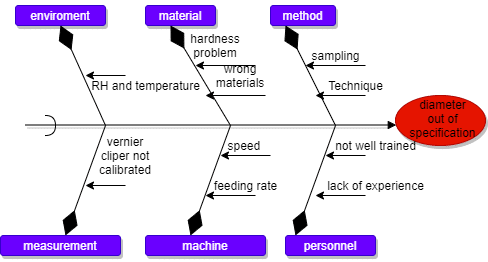
Below is a summary of the 10 most frequently observed audit findings in pharmaceutical manufacturing plants, followed by detailed descriptions, examples, preventive strategies, and relevant industry keywords. Each issue is based on industry reports and regulatory inspection data. Common Audit Finding: 1. Documentation and Record-Keeping Deficiencies: Deficiencies in documentation and records, such as missing or … Read more