1.0 Purpose: To lay down the standard operating procedures for the Collection and Disposal of product waste.
2.0 Scope: This scope is applicable to all the product waste generated during the following activities.
- Granulation
- Compression
- Coating
- Inspection
- Manufacturing and filling
- Packing
- IPQC/QC testing.

3.0 Responsibility:
3.1 Officer concerned department is responsible for the Product Waste; Collection and Disposal.
3.2 All concerned heads of the department and head of Quality Assurance are responsible for ensuring your overall compliance with this SOP.
4.0.Procedure for Product Waste; Collection and Disposal:
4.1 Collection:
- Tablet section: Collect the product waste in a plastic container, and collect all the Powder for product Residue generated in granulation and compression.
- Collect the product Residue or tablets that fall on the floor during compression, coating, inspection, and primary packing.
- Put a “non-recoverable” label on the container as per SOP on “status labeling.”
- At the end of the Shift or changeover, transfer all the “non-recoverable” Residue to a washroom and transfer it in a plastic container bearing the “product waste for disposal label” as per SOP on “status labeling.”
- IPQC: After in-process testing, collects the granules or Tablets in plastic containers wearing the label “product waste for disposal.”
- After the leak test of strip and blister, discard the packing material in the waste Bin and transfer the tablet to a container bearing the label “products waste for disposal.”
- At the end of the Shift transfer all the product waste containers to a washroom, and transfer the product Residue to the plastic container.
4.2 For Oral Hygiene and external preparation:
- Collect the product from vessels for transfer.
- Transporter product in a plastic container and put a “non-recoverable” label on the container.”
- Squeeze out the product from rejected tubes in a plastic container bearing “non-recoverable.”
- At the end of the shift or completion of batch or changeover, transfer all the non-recoverable Residue to the washroom and transfer it in a plastic container bearing the “products waste for disposal” label.
4.3 For Liquid oral preparation:
- Collect the product from vessels/ transfer pumps, pump pipelines, and other equipment as much as possible.
- Transfer the product to a plastic container and put a “non-recoverable” label on the container.
- Open out the product from a rejected bottle in a plastic container bearing “non-recoverable.”
- At the end of the shift or completion of batch or changeover, transfer all the non-recoverable Residue to a washroom and transfer it in a plastic container wearing a “products waste for disposal” label.
- Quality control: after analysis, de-blister the intact strips or blisters and collect the product in a plastic container wearing the label “product waste for disposal.”
- After in-process testing of bulk Mass of oral hygiene /external preparation /liquid oral, pour the Residue into separate plastic containers.
- Squeeze tubes and bottles and pour the contents into the container bearing the label “product waste for disposal.”
- Transfer the containers to a plastic container wearing the label “product waste for disposal” in the washing room.
- Discard the empty tubes, bottles and bottles, and blister strips in a labeled waste Bin for scrap.
5.0 Disposal:
- Transfer the container bearing the “product waste disposal” label in closed condition to a hazardous waste storage room.
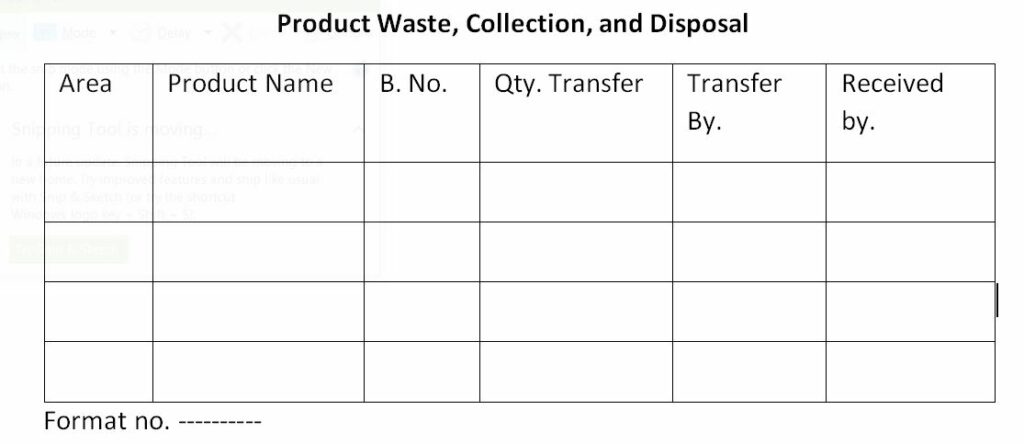
- Record the detail of product waste in the “product waste disposal record” as per the annexure.
- Engineering personnel shall categorize the category of waste As per “List of Biomedical Waste” and fill annexure “logbook for waste disposal by CBWTF” and shall get it approved by the engineering head/ designee before handover the container to the government-approved agency for disposal of waste the annexure and “logbook for dip buried method “will not be maintained at the site.
- Hand over the closed container to the operator of a government-approved agency for disposal and get a sign-on logbook for waste disposal by CBWTF.
Related SOP: SOP on Disposal in Tablet Department
6.0 Abbreviation:
- ETP –Effluent treatment plant.
- Ex -External preparation
- IPQC -In-process quality control
- QA -Quality assurance
- QC -Quality control
- SOP -Standard operating procedure
- BMW -Biomedical Waste
- HCF -Healthcare facility
- CBWTF -Common Biomedical Waste treatment facility
Annexure:

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at Contact@pharmaguddu.com.
