A Dissolution Test is used for in vitro Testing of the Tablets and Capsules. Dissolution provides the product Release info to know the drug delivery. Dissolution apparatus are used throughout the product development life cycle, from Product release to stability testing and study of the product data from product to product. Then after passing or approval from the Quality control and Quality assurance, drugs are sent to markets.

Dissolution test apparatus working principle: The dissolution test apparatus checks how fast a drug dissolves in your body. A tablet or capsule is put in a container that has a medium that is prepared and operated just like the stomach or intestines functions (physiochemical conditions of the body). The container spins or moves around, and samples are taken to see how much of the medicine has dissolved over time. This helps to know how that drug works in a body. It also ensures the quality and efficacy of a product.
Types of Dissolution Apparatus / DT Apparatus:
According to United States Pharmacopoeia and European Pharmacopoeia, Currently, seven types of apparatus are used to identify the characteristics of the solid dosage form. The mostly used apparatus is:
- Basket type (USP Dissolution apparatus 1)
- Paddle type (USP Apparatus types 2)
- Reciprocating cylinder: (USP Dissolution apparatus types 3)
- Flow-through cell:(USP Dissolution Apparatus 4)
Overview of Types of DT Apparatus:
- Basket– for capsules and is operated at 100 RPM
- Paddle– for tablets and operated at 50 RPM
- Reciprocating cylinder for bead type modified release dosage form
- Flow cell– for modified release dosage forms with limited solubility
- Paddle over a disc– for transdermal dosage forms
- Cylinder– for transdermal dosage forms
- Reciprocating disc– for non-disintegrating type oral modified release dosage forms.
A brief explanation of DT Apparatus:
1. Basket type (USP Dissolution apparatus 1):
Basket types apparatus are used for ( for Tablet and Capsule) it consists of the following parts:
A. Cylindrical vessel: Basket types dissolution apparatus Made of borosilicate glass or suitable transparent materials with a hemispherical bottom and a nominal capacity of 1000 ml. The vessel has a flanged upper rim and is fitted with a lid. A cylindrical vessel features a number of openings, one among which is central. 40 mm mesh cloth is generally used in this type of apparatus.
B. Motor: It consists of a Motor with a speed regulator that can maintain the speed of rotation of the paddle within 4% as specified in the individual monograph. The motor is fitted with a stirring element which has a drive shaft and blade forming a paddle.
It Passes the blade through the shaft diameter so that the bottom of the blade and shaft flash at the same time. The shaft is maintained at a position so that its axis is within 2 mm of the axis of the vessels, and the lower edge of the blade is about 23 to 27 mm from the inside bottom of the vessels. The apparatus operates in such a way that the paddle rotates smoothly and without any significant wobble.
C. Water: Dissolution Test apparatus medium bath set to maintain at 36.5° to 37.5 °c during the test. At that time, the bath medium should be in constant motion. The vessel is securely clamped in the water bath in such a way that the displacement of the vibration from other equipment, including the water circulation device, is minimized.
2. Paddle type (USP Apparatus types 2):
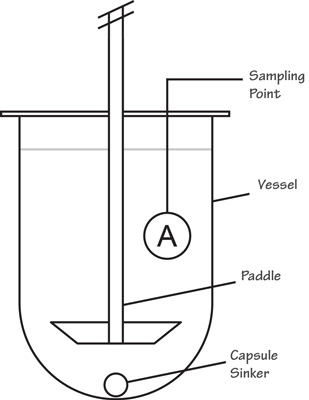
The Paddle-type dissolution apparatus assembly is the same as the basket type except stirring element. The stirring paddle is replaced by the basket. The metallic shaft rotates freely and without a significant vibration.
The basket consists of two components. The top part is attached to the shaft and fitted with free spring clips or other suitable means. This allows the removal of the lower part. This helps in the introduction of the preparation and being examined. It firmly holds the lower part of the basket concentric with the axis of the vessel during rotation.
The lower part of the basket is made by heating( welded ) steam cloth, with a wire thickness of 0.254 mm diameter and 0.381 mm square openings. It turned into a cylinder with a thin rim of sheet metal around both sides, the top and the bottom of the vessels.
The basket may be platted with a 2.5 mm layer of gold for use with the acidic media. It is suggested to maintain a distance of 23 to 27 mm between the bottom of the vessels and the basket during the test.
The method used in the Paddle types apparatus: Operate the paddle types Dissolution test after placing 1 tablet or capsules in the fixed amount of dissolution medium in the vessel at 37 ± 0.5°C.
Then firstly, Withdraw a portion of the testing solution from the midway zone. Secondly, withdraw from between the surface of the dissolution medium, then from the top of the rotating basket or blade, and finally from the vessel wall (not less than 1cm) within the time interval.
3. Reciprocating cylinder: (USP Dissolution apparatus types 3):
A Reciprocating cylinder was developed to mimic the gastrointestinal tract. It consists of a set of cylindrical, flat-bottomed glass vessels, a set of glass reciprocating cylinders with inert fitting, and a screen at the top and bottom of the cylinders.
Reciprocating cylinder Procedure: Place the fixed amount of dissolution medium in each vessel of the Dissolution test apparatus at 37 ± 0.5°C. Then operate it with the addition of 1 dosage form in each of the six reciprocating cylinders. The cylindrical vessel moves through a distance of 9.9 to 10.1 cm, while up and down movements.
Then firstly, withdraw a portion of the testing solution from the midway zone and secondly from between the surface of the dissolution medium. Then finally, from the bottom of each vessel within the time specified perform the analysis as given in the individual monograph.
4. Flow-through cell:(USP Dissolution Apparatus 4):
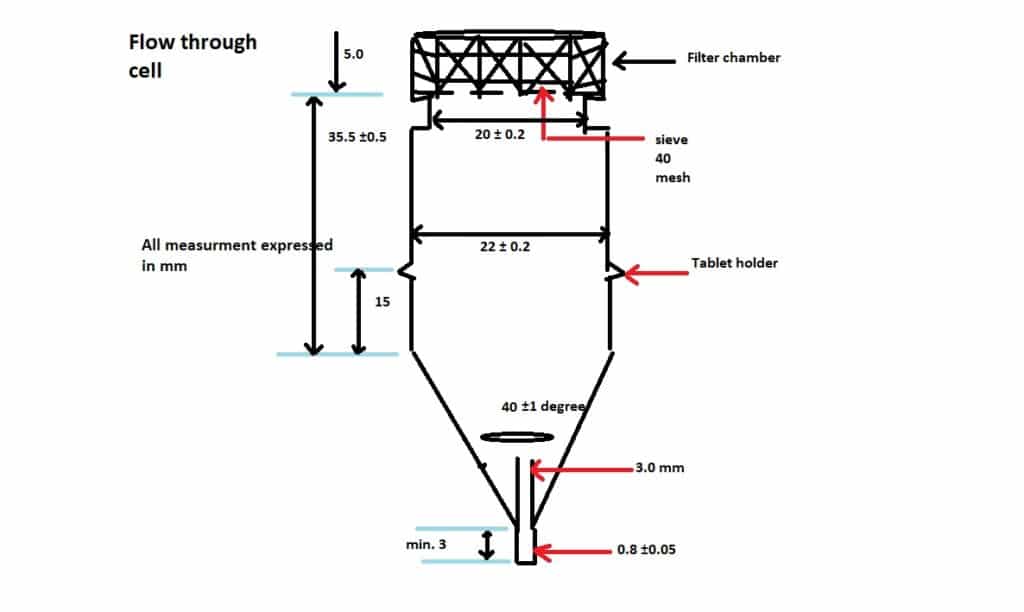
The Flow-through cell is Most useful for low-solubility drugs, implants, and suppositories. Flow-through cells are both open and closed-type systems. The open system used a fresh medium that pumped through the cell and fraction at every 30 to 60 minutes. This results in a high fraction volume. This type of system is valuable for poorly soluble drugs or pH changes to be performed.
On the other hand, in a closed system medium is pumped through the circle but without the use of the fresh medium. This system is used when a drug product has a very low strength especially when performed in low volume. The pump delivery ranges between 240 and 960 ml every hour.
Flow-through cell Procedure: For this, Maintain a dissolution medium at 37 ± 0.5 °C. Place 1 dosage form unit through the bottom of the cell on top of the beads. Then collect the solution by fractions at each of the times as specified and perform the analysis as given in the individual monograph.
5. Paddle over disc:
It is similar to a Paddle apparatus, But it comes with the addition of a stainless steel disc assembly. The temperature is maintained at 32 ± 0.5°C with a standard volume of 900 ml. During the test, maintaining a distance of 25±2 mm between the paddle blade and the surface of the disc is necessary to minimize any dead volume between the disc assembly and the bottom of the vessel.
Paddle over disc Procedure: Place the disc assembly flat at the bottom of the vessel and attach the system to the disc with a suitable adhesive.
Operate the apparatus with a fixed amount of dissolution medium in the vessel at the medium of 32 ± 0.5 °C. Then firstly, withdraw a portion of the testing solution from a midway zone and between the surface of the dissolution medium and the top of the blade. It should not be less than 1 cm from the vessel wall within the specified time. Perform the analysis on each sample solution as given in the individual monograph.
6. Cylinder:
It is similar to the basket type except for the basket and shaft. It was replaced with a stainless steel cylinder stirring element to maintain the temperature at 32 ± 0.5 °C. Ensure the distance of 25 ± 2 mm is maintained between the inside bottom of the vessel and the cylinder during the test.
Cylinder Apparatus Procedure: Remove the protective liner and place the piece of cuprophan on the adhesive side of the system, and down the covered side of cuprophan on a clean surface and apply a suitable adhesive on cuprophan borders and then Dry it for 1 minute. Finally, Remove trapped air bubbles by pressing the cuprophan covering and Rotating the cylinder at a specified rate.
Firstly withdraw a portion of the testing solution from a zone midway and between the surface of the dissolution medium. Secondly, withdraw from the top of the rotating cylinder. It should not be less than 1cm from the vessel wall. Withdraw the sample within the specified time. Then perform the analysis as given in the individual monograph.
7 Reciprocating disc :
The reciprocating cylinder is also called a reciprocating holder. It consists of a volumetrically calibrated and tared solution, a motor, and a drive assembly to reciprocate the system vertically. The solution containers are partially immersed in a suitable water bath, at a temperature of the containers at 32 ± 0.5 °C.
Medium use for Dissolution Test:
If the medium is a buffered solution, then adjust the pH within 0.05 units of the pH specified in the monograph. The dissolution medium should be De-aerated prior to testing.
Dissolution Time:
The test may be concluded in a shorter period as per the single time specification is given in the monograph if the requirement for the minimum amount dissolved is met If two or more times are specified. Then the specimen is to be withdrawn only at the stated times, within a tolerance of ± 2%.
Test Method for Dissolution Apparatus:
Place the stated volume of dissolution medium, reform dissolved air, into the apparatus vessel, then unit the whole part of the apparatus and warm the dissolution medium between 36.5 to 37.5°C.
Use one tablet or capsule when basket-type apparatus is used to allow the tablet or capsule to sink to the bottom of the vessel former to the rotation of the paddle.
During the test, tablets and capsules may float, so use a wire of glass helix. Place it horizontally at the bottom of the vessels. Care should be taken to ensure that air bubbles are excluded from the surface of the tablets or capsules.
When paddle-type apparatus is used, place the unit dose (tablet, or capsule) in a dry basket at the start of each test. Make sure to lower the basket into position before rotation. Operate the apparatus with the speed of rotation specified in the individual monograph.
The volume of Dissolution medium:
Add a volume of dissolution medium equal to the volume of sample withdrawn in the case of single sampling. Perform the analysis as given in the individual monograph. Repeat the whole process five times, where two or more tablets or capsules are directed to be placed together in the apparatus and carry out six replicate tests.
Use the membrane filter disc to filter the sample solution with an average pore diameter not greater than 1.0 microns. Make sure to discard the first few ml. drops of the filtrate. Then calculate the amount of the dissolved active ingredient in the solution as a percentage of the stated amount. Where two or more tablets or capsules are placed together,
Suppose the results do not conform to the requirements at stage S1 given in the accompanying acceptance tablets. Continue testing with additional tablets and capsules through stages S2 and S3 unless the result conforms at stage S2.
Remove the capsule shells, remove the contents of not to be less than 6 capsules as completely possible, and dissolve the empty capsules in a specified volume of dissolution medium. Perform the analysis as given in the individual monograph.
Importance of Dissolution Testing in Pharmaceuticals
Dissolution testing is also done to ensure the consistency of products from batch to batch. For drugs or dosage forms to be efficacious, the active ingredients(API) must be Absorbed into our systemic circulation so that they can act on site. This process helps achieve the bio-availability of drug substances, and it involves two steps: Dissolution and Absorption.
Dissolution is the process of extracting the active ingredients of drugs into a solution. For this, we use the dissolution test apparatus. So types of DT apparatus used are explained above:
Summary
The Dissolution Test is Important for in vitro testing tablets and capsules, providing essential drug delivery information. Dissolution apparatuses play an important role in product development, covering stages from release to stability testing. The process involves using different types of apparatus, such as a Basket, Paddle, Reciprocating Cylinder, Flow-through Cell, Paddle over Disc, Cylinder, and Reciprocating Disc. From above each apparatus has its purposes and uses and operates under defined conditions.
For example, the Basket apparatus is suitable for tablets and capsules, operating at 100 RPM. The Paddle apparatus, at 50 RPM, is ideal for tablets. Reciprocating Cylinder mimics the gastrointestinal tract, while Flow-through Cell is useful for low-solubility drugs. Paddle over Disc combines a Paddle with a stainless steel disc for specific testing.
Dissolution testing is essential for maintaining product consistency across batches and facilitating the absorption of the active components for effectiveness. It requires maintaining exact parameters, such as the temperature and dissolution media, and calculating the proportion of the dissolved active component. This comprehensive procedure ensures the bioavailability of medication ingredients, which enhances the potency of pharmaceuticals.
Frequently Asked Questions
Ans: USP Chapter 711 demonstrates the Dissolution
Ans: It is the time required for a drug to dissolve in a medium.
Ans: Disintegration is the time required to fragment the drugs
Ans: The dissolution profile is the in-vitro study to know the percentage of the drug dissolved at different time intervals.
Ans: S1, S2, and S3
Ans: S1-6 unit, S2-12 unit, (total 12 unit including 6 unit from S1) S3- 12 unit total 24 unit including a unit from S1+S2+S3
Ans: 6 units for precision

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
