The limit test for iron refers to a type of analytical chemistry test used to determine the concentration of iron in a sample. This test is often performed in the laboratory as part of a larger suite of tests to determine the chemical composition of a substance.
In a limit test for iron, a known volume of the sample is treated with a reagent that reacts with iron to produce a characteristic color or other detectable signals. The intensity of the color or signal is then measured and used to calculate the concentration of iron in the sample. The test may have a specified limit of detection or limit of quantitation, which refers to the minimum concentration of iron that can be accurately detected or measured using the method.
Definition
Definition of limit test iron is defined as a quantitative test to identify and control a small quantity of impurity which is present in the substance.
Principle:
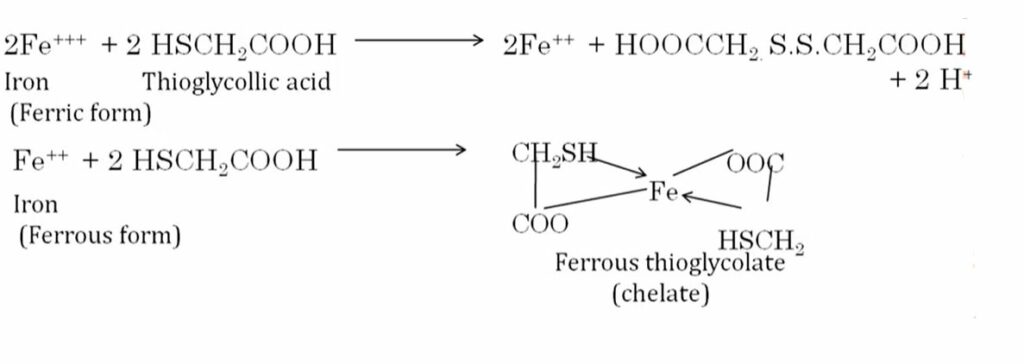
The limit test for iron is based on the formation of the purple color by reacting the iron with thioglycolic acid in a solution buffered with ammonium citrate. the color produced is compared with a standard color containing a known amount of iron (0.14 mg of Fe).
Apparatus Required
- Nessler Cylinder
- Glass rod
- Pipette
- Stand
Reagent Required
- Citric acid
- Thioglycolic acid
- Ammonia
The roles of Reagents
Thioglycolic acid– First Thioglycolic acid converts Fe+3 to Fe+2 and the second function is the formation of purple color salt.
Ammonia– Provides an alkaline medium for reaction.
Citric acid– Citric acid prevent the formation of a precipitate of iron with ammonia.
Reaction equation
Procedure for Limit test for Iron
The practical approach for limit test for iron is as follows the procedure:
| Standard Solution | Test Solution |
| Take a Nessler cylinder and add 2 ml of a standard solution of iron (20 ppm of Fe) | Take sample |
| 40 ml of D/W water | 40 ml of D/W water |
| 2 ml of 20% w/v citric acid (iron free) | 2 ml of 20% w/v citric acid (iron free) |
| 2 drops of thioglycolic acid | 2 drops of thioglycolic acid |
| Made the solution alkaline with ammonia and adjust the volume by adding water up to 50 ml | Made the solution alkaline with ammonia and adjust the volume to 50 ml |
| Mix the solution and keep aside for 5 minutes to stand | Mix the solution and keep aside for 5 minutes to stand |
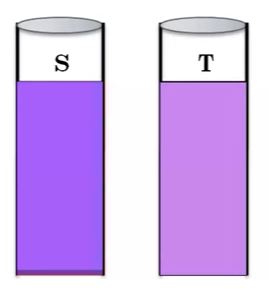
Observation
The purple color produced in the sample solution should not be greater than the standard solution. If the color intensity of the test solution is less than the standard solution then it complies as per IP.
Different methods employed for limit test for Iron
There are several methods that can be used to perform a limit test for iron, including:
Titration: This method involves adding a known volume of a reagent that reacts with iron to a known volume of the sample, and then measuring the volume of reagent required to react with all of the iron present in the sample. This method is often used when the concentration of iron in the sample is not known in advance, or when the sample contains other contaminants that may interfere with the analysis.
Spectrophotometry: This method involves measuring the absorbance or transmission of light by the sample at a specific wavelength. The absorbance or transmission is proportional to the concentration of iron in the sample and can be used to determine the concentration of iron using a standard curve or calibration curve.
Inductively coupled plasma mass spectrometry (ICP-MS): This method involves vaporizing the sample and ionizing the resulting atoms or molecules using an inductively coupled plasma. The ions are then separated based on their mass-to-charge ratio and detected using a mass spectrometer, allowing for the precise measurement of the concentration of iron in the sample.
Atomic absorption spectroscopy (AAS): This method involves measuring the absorbance of light by the sample at a specific wavelength. The absorbance is proportional to the concentration of iron in the sample and can be used to determine the concentration of iron using a standard curve or calibration curve.
Atomic emission spectroscopy (AES): This method involves measuring the emission of light by the sample at a specific wavelength. The emission is proportional to the concentration of iron in the sample and can be used to determine the concentration of iron using a standard curve or calibration curve.
Related: Preparation and Standardization of 0.1 N HCl

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
