MACO and NOEL calculations have great importance in the pharmaceutical industries. NOEL refers to “No observed effect level” and MACO is a “Maximum allowable carryover”. NOEL is used to determine MACO during cleaning validation.

NOEL Calculation:
NEOL is calculated by using LD50 and average. Adult dose.
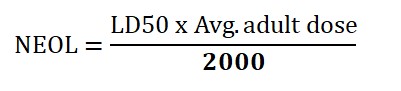
LD50 is a lethal dose (it may vary), considered an adult’s average. Weight is 70kg, and 2000 is content. For example, if an LD50 of any drug product is 250mg/kg. then NOEL calculation is
NEOL= 250×70/2000= 8.75mg.
MACO (or MAC) Calculation:
Method 1:
If the MACO of the previous product is in the next batch. the calculation is as follows:
In this equation, we have a previous product, and a next product by taking into consideration of therapeutic dosage of the drug product in which the API is used.
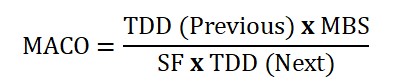
MACO: Maximum Allowable Carryover
TDD previous: Therapeutic Daily Dose of previous product
TDD next: Therapeutic Daily Dose of next product
MBS: Minimum batch size for the next product
SF: Safety factor (normally 1000 is used in calculations based on TDD)
For Example: Product A has been cleaned out. The product A has a standard daily dose of 10mg and the batch size is 200 kg. The next product B has a standard daily dose of 250 mg and the minimum batch size is 50 kg. Both A and B are administrated orally and SF is set to 1000. Calculate the MACO for A in B. So by using the formula:
MACO= 10 (mg) x 50,000,000 (mg)/ 1000 x 250 mg=2000 mg so Result will be 2000 mg (2g).
Method 2:
The second method of calculation takes toxicological data into consideration. From the NOEL value, MACO is calculated as:
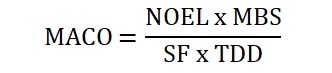
Whereas NOEL is 8.75mg.
MBS is the Minimum batch size for the next product
SF is the Safety factor.
TDD is the largest normal daily dose for the product
For example: if the total daily dose of a product is 350mg and the batch size is 100 kg, our NOEL is 8.75 mg. Then MACO can be calculated as follows:
MACO= 8.75(mg)x100000000/1000×350(mg)= 250mg or 0.25 gm.
Acceptance criteria:
This is the value of allowable residue of the previous product in the next product. Since the residue of the previous batch is contaminated in the next product, it is necessary to limit such carryover into the next product. The maximum limit that is permitted is called the MACO.
What NOEL and MACO prove:
By using NOEL and MACO, we can find out the quantity of a drug that can not be carried out over to the next batch. As studies above 250mg /kg LD50 should not be over 0.25gm in the next batch as per above the batch has 350mg daily dose and 100 kg batch size.
Scope of MACO in pharmaceuticals:
MACO and NOEL Calculation are widely used for determining acceptance criteria, cleaning levels, Determination of the amount of residue present, and cleaning validation protocol.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
