Aim: To prepare and standardize 0.5 M sulfuric acid (0.5 M H2SO4).

Principle:
1. This is carried out by aqueous acid-base titration based on a neutralization reaction.
2. This titration takes place between sulphuric acid (H2SO4) and sodium carbonate (Na2CO3).
3. Sodium carbonate, anhydrous is a primary standard substance that reacts with a secondary standard substance, H2SO4.
4. Anhydrous sodium carbonate is a strong base whereas sulphuric acid is a strong acid.
5. Hence, it is strong base-strong acid (SB – SA) type of neutralization titration.
6. Methyl red solution is used as an indicator. It is a preferred indicator for this type of neutralization titration.
Requirements for Preparation and Standardization of 0.5 M Sulfuric acid:
1. Apparatus: Beaker, conical flask, pipette, pipette filler, burette, measuring cylinder, burette stand and holder, glass funnel, white tile, bench mat.
2. Chemicals: Sodium carbonate solution, concentrated sulphuric acid, methyl red indicator solution.
Theory:
(A) Sodium carbonate (Primary standard):
(B) Sulphuric acid (Titrant):
1. Molecular formula: H2SO4
2. Molecular weight: 98.079
3. Equivalent weight: 49.03
4. It is a diprotic acid and contains two replaceable hydrogen ions, therefore its equivalent weight is half of its molecular weight.
5. Chemical names: Oil of vitriol. This name was given because pure sulphuric acid is a viscous clear liquid, like oil.
Description: It is clear, odorless, pungent-ethereal, colorless to slightly yellow viscous liquid, a highly corrosive strong mineral acid, and a powerful protonating agent. It is hygroscopic, readily absorbing water vapors from the air. The 98% grade is more stable in storage, and is usually referred to as “concentrated sulphuric acid”.
Safety:
- The reaction, specifically a hydration reaction, between sulphuric acid (H2SO4) and water (H2O) is known to be highly exothermic, meaning it releases a significant amount of heat. Consequently, when diluting sulphuric acid, it is essential to follow a specific procedure for safety purposes.
- Dilution should always be carried out by adding the acid to the water, rather than the water to the acid.
- Sulphuric acid at a high concentration can cause very serious damage up; contact. It burns the cornea and can lead to permanent blindness if splashed onto the eyes.
- Sulphuric acid must be stored carefully in containers made of non-reactive material (such as glass). Solutions equal to or stronger than 1.5 M are labeled as “CORROSIVE”, while solutions greater than 0.5 M but less than 1.5 M are labeled as ( “IRRITANT”. However, even the normal laboratory “dilute” grade (approximate) 1 M, 10%) solutions produce a charing effect by dehydration. if left in contact for sufficient time.
- The standard first aid treatment if acid spills on the skin is irrigation with a large quantity of water for at least ten to fifteen minutes. Contaminated clothing was removed immediately and the underlying skin was washed thoroughly.
Preparation of Reagents/Solutions:
0.5 M sulphuric acid Preparation: Add slowly, with stirring, 30 ml of concentrated sulphuric acid to about 1000 mL of water, and allow to cool at 25°C.
Standardization of 0.5 M H2SO4:
- Weigh accurately about 1.5 g of anhydrous sodium carbonate (which is previously heated at about 270°C for 1 hour).
- Dissolve 1.5 g of anhydrous sodium carbonate in 100 mL of distilled water.
- Add 0.1 mL of methyl red solution as an indicator.
- Gradually add 0.5 M sulphuric acid from a burette into the solution while stirring continuously until the solution turns slightly pink.
- Next, heat the solution to a boiling point, allow it to cool down, and then proceed with the titration process.
- Heat the solution once again until it reaches the boiling point, and proceed to titrate it further as required until the faint pink color remains unaffected by the continued boiling.
- Related Topic:
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
Reaction:
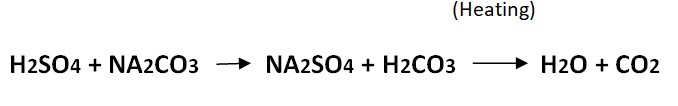
Strong acid (Sulphuric Acid) reacts with a strong base (Sodium carbonate) to form N2SO4 salt and carbonic acid which further form water molecules and carbon dioxide on heating.
Factor calculation:
1 mole of H2SO4= 1 mole of sodium carbonate
1000 mL of 1M H2SO4= 106 g of Na2CO3
1000 mL of 0.5 M H2SO4= 53 g of Na2CO3
1 mL of 0.5 M H2SO44= 0.053 g of Na2CO3
1 mL of 0.5 M sulfuric acid is equivalent to 0.05299 g of Na2CO3
Calculation:
Equivanalt factor method:
1 mL of 0.5 M H2SO4= 0.05299 g of Na2CO3
X mL of y M H2SO4 = 1.5 g of Na2CO3
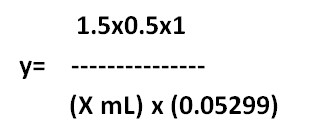
y= ………M
Formula Method:
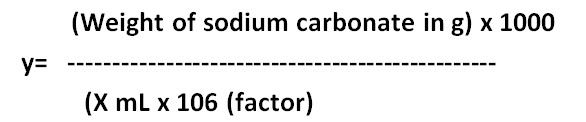
y= ……M
Result: The molarity of sulfuric acid solution (Prepared, 0.5 M) was found to be …….M

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
