Serialization in pharmaceuticals plays a vital role in securing product information, counterfeit, and falsified medicine that puts world public health at risk. These medicines are also not only ineffective but actively dangerous, inflicting adverse reactions or even death. What is more, these counterfeit medicines are also factory-made in multiple locations, e.g., chemical synthesis in one country, the addition of fillers in another, and packaging in third sites making them tough to trace and deduct from the supply chain.
So to reduce the proliferation of counterfeit medication within the U.S., the Drug Quality and Security Act (DQSA) was signed into law in November 2013. Title II of DQSA outlines steps to make the associated electronic system spot and trace prescribed drugs as they’re factory-made and distributed. These rules apply to makers, repackagers, wholesale distributors, dispensers, and third-party supplying suppliers.
Here are many basics to assist you in understanding the relevant regulatory problems and, therefore, the serialization method.
Serialization in Pharmaceuticals, What it does?
Serialization in pharmaceuticals involves tracing the product’s origin and batch details, like the Batch number and Expiration date via the unique serial number from the manufacturer to consumers.
The manufacturer will produce a serial number for products and apply it on different batches via printing on labels and cartons with a barcode system. The manufacturer keeps all the records of these serial numbers for every batch after the manufacturing or packing of a product.
The units will then be tracked through its entire provide chain — from production to retail distribution to the ultimate dispensation to the patient. The DQSA mandates that every pharmaceutical sold within the USA should be serialized at each marketable unit level.
Serialization Process Flow:
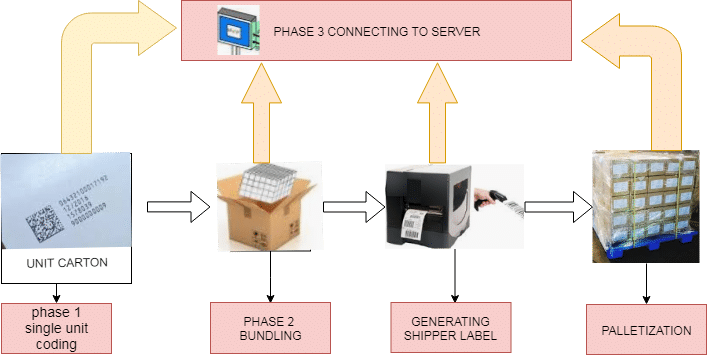
The Food and Drug Administration guidelines recommend the employment of standard numerical identifiers (SNRIs); during this, the serialized national drug code (sNDC), which is the NDC combined with a singular serial range. the entire process of Serialization in pharma has involved the following stages:
- First processing randomly and subsequently of serialization code, the manufacturers print a 2D Barcode on each label generated during the process.
- Each label scans to determine which small unit goes into which final pack because the lower unit is not visible(e.g., first carton print with a 2D barcode then after scan goes to which shipper) and a number of shippers in each pallet.
- It is speculated by massive distributors that the Food and Drug Administration will shortly need the gathering of serialization data and verification that serial numbers were in every bundle, shipper, and pallet, and ultimately that units were sent to every distributor.
- To possess this info without reopening each shipping case, it needed that the maker aggregates the data throughout the method.
- This data can then be sent electronically in an associate advance shipping notice (ASN). All parties should retain the data for the Food and Drug Administration audits.
- By 2023, Pharmaceutical company manufacturers should also implement an associated electronic system capable of tracing all packages of pharmaceuticals through the complete distribution chain.
Key challenges in Serialization:
Serialization in pharmaceuticals causes different problems at different levels during the pharmaceutical manufacturing process:
- Redesign of packaging: Many manufacturers face this problem because manufacturers need to redesign labels significantly leads to the re-design also of packaging structure and elements.
- Reduce production efficiency: Due to its complected and long process like label printing, scanning at every stage will slow down the packaging line. So to compensate, the manufacturer needs to require more manpower.
- Data storage: Data storage and its generation, storage, and capture of lots of serial numbers are key challenges for the IT department.
- Lack of trained personnel: The serialization process required a well-trained team of experts from IT, processing/ maintenance, packaging, and QA to operate a smooth operation.
- Cost: Serialization requires more expenditures to operate, renew, or update existing machinery, software, and hardware.
Benefits of Serialization
This data information may be examined and used to improve supply chain and sales visibility, resulting in more accurate demand forecasting and inventory management, more accurate order fulfillment and delivery, and more effective and efficient product recall management and returns processing.
Data from serialization may be utilized to enhance pharmaceutical management systems.
It may also be used to streamline and automate procurement, invoicing, warehouse performance measurement, and other pharma supply chain activities.
Read Also: ICH Guidelines in Pharmaceutical
FAQs
serialization is the unique identification number assigned on each pack of cartons and bottles, a globally unique number is assigned to each batch of finished products in the form of a two-dimensional code known as Datamatrix.
The track system makes it easy to understand the location of any product items and Trace is to understand the history of products that come in contact with the item in the supply chain.
The track and trace system can determine the current and past location of unique items, in response to an increase in the number of recalled products from the market the track and trace system is employed in the pharmaceutical, it is good in traceability through radio frequency identification and barcode.
RFID is radio frequency identification, a new technic is a part of supply chain management in international pharmaceutical production and distribution. RFID is employed to counterfeit drug products which are highly challenging for the government, pharmaceutical companies, clinical, and to patients.
The Global Location Number (GLN) identifies the nation of origin. Serial Number is a unique identifier for each product under the specific GTIN Global Trade Item Number (GTIN) to identify the kind of product
Lot information is used to distinguish across manufacturer-specific batches of items from the same production run.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].

Serialization will do wonders in alleviating the growth of counterfeit drug market. Thanks for sharing.