The Friability test helps measure the powder lost from tablet surfaces during handling and transport. This test is typically done on uncoated tablets.
The parallel to friability test, tablets hardness, is also tested to know the strength of tablets which is more important for drugs to withstand throw-out their lifecycle. However, hardness is not only a parameter for tablet strength. Sometimes compressed tablets may lose their crown portion or cap on attrition.
So it is vital to do a friability test to know the percentage of loss powder, which is calculated by using a formula that we have discussed below in this article. As per the formula, the limits of the friability test shall not be more than 1.0%.
Friability Test Apparatus Specifications (MOC):
The friability apparatus consists of a rounded shape drum made of transparent synthetic polymer with a smooth internal surface. It is specially designed to minimize static charge. The apparatus includes a single drum or two drums that are horizontally attached to the axis of the apparatus. The Roche friability apparatus is commonly used for the friability procedure.
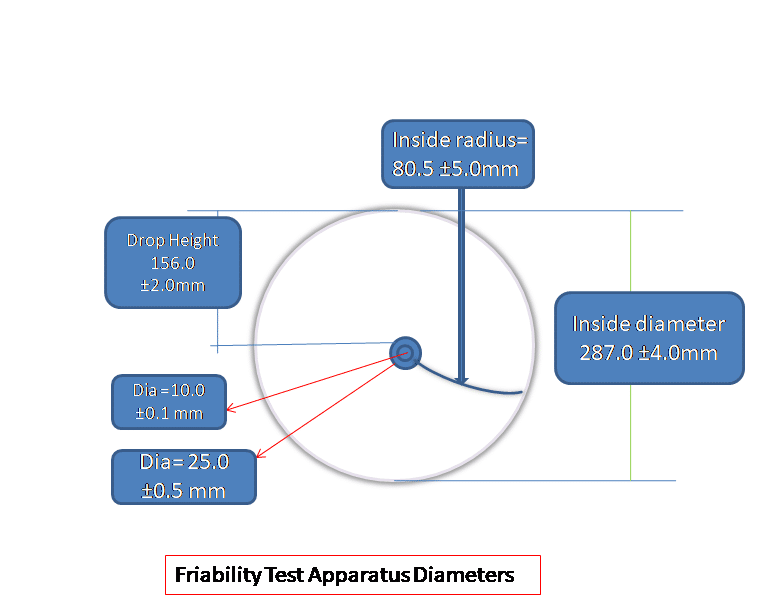
Note: Friability Apparatus shall comply with USP <1216>, EP<2.9.7>, and other pharmacopeias.
Friability apparatus Diameter as per Pharmacopeias:
- Internal Diameters: Range from 283.0 to 291.0 mm (with a mean of 287.0 ±4.0mm).
- Depth: Ranges from 36.0 to 40.0 mm (with a mean of 38.0 ±2.0 mm).
- Outer Diameters: The outer diameter of the central ring ranges from 24.5 to 25.5 mm (with a mean of 25.0 ±0.5 mm).
- The Drop height of the friability apparatus is approximately 6 inches or 156±2.0 mm.
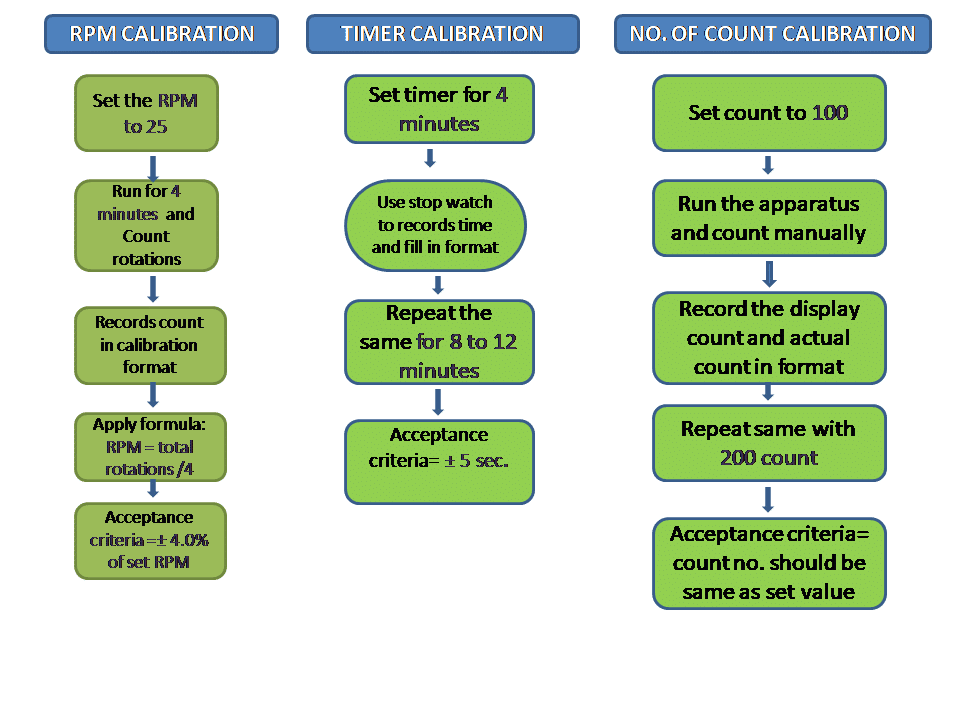
- Friability Apparatus RPM (revolution/ minutes) is set at 24 – 26 (with a mean of 25 ±1).
- Total Revolution / Test: 100 Revolutions in 4 Minutes.
Responsible person to perform test:
- Production officer/ executive
- IPQA officer
- Quality control officer
Friability Test procedure:
- Collect an equal number of tablets from both sides of the chute and affix a status label on them. Take tablets weighing equal to or less than 6.5 g if the unit weight is 650 mg or less.
- If the tablets have a unit weight exceeding 650 mg, collect ten tablets as a whole sample.
- Check the calibration status before starting the operation.
- Before the test, dedust all tablets, record their weights, and place the required quantity into the drum.
- Set all parameters (revolution time) and start the test.
- Upon completion, dedust all the tablets and re-weigh them.
- Proceed to apply the formula to calculate the friability.
- If the result exceeds the accepted limits, repeat the test three times and calculate the mean.
Note: Proper de-dusting of tablets is to be done before and after the test to avoid false results.
Important: If the shape or size of an uncoated tablet is causing irregular Tambling, the friability drum can be shifted 10º from the main position.
Friability test Formula:

w1= Initial weight of tablets or weight before a test.
w2= Final weight of tablets or weight after the test.
Friability Limits: According to USP, IP, and BP, It should be Not more than 1.0%.
Calibration of Friability test Apparatus:
Conclusion:
While using the friability apparatus, We can quickly determine the powder loss from the uncoated tablet core surface. Friability tests are beneficial to maintain the quality of solid oral dosage forms (tablets) and further help to know about product stability, which is required for further processing and transportation.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
