Difference Between Validation, Calibration, and Qualification in Pharma
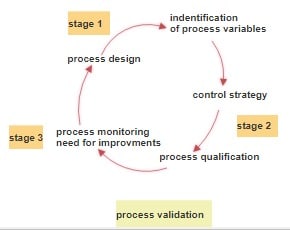
There is often confusion surrounding the terms validation, calibration, and qualification within the pharmaceutical industry. Let’s explore their differences with explanatory examples. Difference among Validation, Calibration, and Qualification 1. Qualification: Qualification is the process of planning, conducting, and documenting test results performed on equipment to confirm its operational capability. It demonstrates that the equipment will … Read more