Performance Qualification of Automatic Capsule Filling Machine defines the test procedures, documentation, references, and acceptance criteria to establish that the performance of the equipment shall meet the predetermined acceptance criteria.

| SERIAL NO. | ITEM DESCRIPTION |
| 1.0 | PROTOCOL APPROVAL |
| 2.0 | OVERVIEW: |
| 2.1 | Objective |
| 2.2 | Purpose |
| 2.3 | Scope |
| 2.4 | Responsibility |
| 2.5 | Execution Team |
| 3.0 | GENERAL CONSIDERATION/PREREQUISITE |
| 4.0 | REVALIDATION CRITERIA |
| 5.0 | PERFORMANCE QUALIFICATION PROCEDURE |
| 5.1 | Methodology |
| 6.0 | ENVIRONMENTAL CONDITION |
| 7.0 | CAPSULES PARAMETERS |
| 7.1 | In-process checks |
| 7.2 | Uniformity of weight |
| 8.0 | DEFICIENCY AND CORRECTIVE ACTION (S) REPORT (S) |
| 9.0 | Annexure (s) |
| 10.0 | PERFORMANCE QUALIFICATION FINAL REPORT |
| 10.1 | Summary |
| 10.2 | Conclusion |
| 10.3 | Final report approval |
| 1.0 | PROTOCOL APPROVAL: |
This performance qualification protocol for the Automatic capsule filling machine has been reviewed and approved by the following persons:
| FUNCTION | NAME | DEPARTMENT | SIGNATURE | DATE |
| PREPARED BY | QUALITY ASSURANCE | |||
| REVIEWED BY | PRODUCTION | |||
| REVIEWED BY | QUALITY CONTROL | |||
| APPROVED BY | QUALITY ASSURANCE |
| 2.0 | OVERVIEW : |
| 2.1 | OBJECTIVE: |
| The objective of Automatic filling capsules PQ is to develop and execute, the document verification of all aspects of the equipment that can affect product quality. To make an impact assessment of the critical components of the equipment on the material To establish, check, and document the performance of equipment in the established/predetermined operating ranges. | |
| 2.2 | PURPOSE: |
| The purpose of this protocol is to verify that the equipment produces the desired output. Performance qualification of the equipment is planned after successfully completing the installation Qualification and operational qualification of the automatic capsule-filling machine. The performance of the Automatic capsule-filling machine is verified by charging the hopper with the blend and verification of physical parameters of the tablets. The filled capsule was collected at different stocks and varying other parameters. |
| 2.3 | SCOPE: |
| The protocol shall define the test procedures, documentation, references, and acceptance criteria to establish that the performance of the equipment shall meet the predetermined acceptance criteria. The Scope of this protocol is limited to the performance qualification of the Automatic capsule filling machine at XYZ Pharmaceuticals, and the Capsule filling area. Once the performance qualification of the Automatic capsule filling machine has been completed successfully, the equipment shall be released for the production trial | |
| 2.4 | RESPONSIBILITY: |
| The following shall be responsible; Quality assurance officer/Executive – For Preparation of Protocol /Execution. Production Head – For execution support. Quality Control head – To analyze the process. Quality Assurance Head – For adequacy and final approval. |
| 2.5 | EXECUTION TEAM: |
| The execution team is responsible for the execution of performance qualification of Automatic capsule filling machine. The execution team comprises of: |
| DEPARTMENT | DESIGNATION | NAME | SIGNATURE | DATE |
| PRODUCTION | ||||
| QUALITY CONTROL | ||||
| QUALITY ASSURANCE |
| 3.0 | GENERAL CONSIDERATION/PREREQUISITE: |
| 3.1 | Approved SOP of the equipment shall be available. |
| 3.2 | The impact analysis of the equipment shall be recorded in the summary sheet. |
| 3.3 | The installation and operational qualification of the equipment shall be successfully completed before the execution of the performance qualification. |
| 3.4 | All the deficiencies and discrepancies related to the equipment that affect the product quality and corrective action taken shall be recorded in the appropriate section of the protocol. |
| 3.5 | The analytical test results and other reports related to the equipment shall be attached to the performance qualification of the equipment and finally verified. |
| 3.6 | After completion of PQ activities, equipment shall be cleaned as per respective cleaning SOPs and released for manufacturing. |
| 4.0 | REVALIDATION CRITERIA: |
| The machine shall be revalidated if: | |
| There are any major changes, which affect the performance of the equipment. After major changes in the components of the equipment. |
| 5.0 | PERFORMANCE QUALIFICATION PROCEDURE: |
| 5.1 | METHODOLOGY: |
| The principle of the Automatic capsule-filling machine is to fill the capsule with a blend within the capsule specification limit and at a specified speed. This machine can be used for filling of capsules at the desired weight so as to achieve post-filled capsule tests such as locking length, Appearance, and weight variation within permissible limits. Blend shall be charged to the hopper of the Automatic capsule filling machine with the help of a lifting & posting device. Filled capsules are collected in the SS IPC. The samples shall be collected at 60, 80, and 106 SPM. |
6.0 ENVIRONMENTAL CONDITION:
| Temperature(Limit: 25 ± 20 C) | Humidity (RH) (Limit: NMT 50 ± 5%) | ||
| MAX | MIN | MAX | MIN |
INFERENCE:
————————————————————————————————————————————-
————————————————————————————————————————————-
————————————————————————————————————————————-
————————————————————————————————————————————-
————————————————————————————————————————————-
Checked by:
Date:
7.0 CAPSULE PARAMETERS:
| Sr. No. | Parameters | In-Process Specifications | No. of Capsules Req. |
|---|---|---|---|
| 1 | Appearance | Scarlet / Scarlet cap/body Empty hard gelatin capsules of size ‘2’ | 100 |
| 2 | Weight of 20 filled Capsules | 7.10 g ± 2 %(6.96 to 7.24 g) | 20 |
| 3 | Weight of 20 Empty capsules | 1.20 g ± 2 %(1.18 to 1.22 g) | |
| 4 | Uniformity of weight | 355 mg + 5.0 % | 30 |
| 5 | Locking length | (17.2 to 18.0 mm) | 04 |
7.1 IN-PROCESS CHECKS:
| At 60 SPM | |||||||||
| Date | Time | Appearance | Locking length (17.2 to 18.0 mm) | Average wt.Of filled 20 capsules | DoneBy | Ckd. By | |||
| At 80 SPM | |||||||||
| Date | Time | Appearance | Locking length (17.2 to 18.0 mm) | Average wt.Of filled 20 capsules | DoneBy | Ckd. By | |||
| At 106 SPM | |||||||||
| Date | Time | Appearance | Locking length (17.2 to 18.0 mm) | Average wt.Of filled 20 capsules | DoneBy | Ckd. By | |||
7.2 UNIFORMITY OF WEIGHT:
Limit = none of the capsules should be deviated by ±7. 5 %
NMT 2 capsules should be deviated by ±10 %
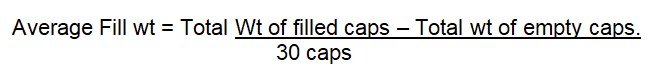
| 60 SPM | 80 SPM | 106 SPM | ||||||||||||
| Sr.No. | Wt of Filled caps. in mg | Wt of empty caps. in mg | Fill wt of caps. In mg | Wt of Filled caps. in mg | Wt of empty caps. in mg | Fill wt of caps. In mg | Wt of Filled caps. in mg | Wt of empty caps. in mg | Fill wt of caps. In mg | |||||
| 1. | ||||||||||||||
| 2. | ||||||||||||||
| 3. | ||||||||||||||
| 4. | ||||||||||||||
| 5. | ||||||||||||||
| 6. | ||||||||||||||
| 7. | ||||||||||||||
| 8. | ||||||||||||||
| 9. | ||||||||||||||
| 10. | ||||||||||||||
| 11. | ||||||||||||||
| 12. | ||||||||||||||
| 13. | ||||||||||||||
| 14. | ||||||||||||||
| 15. | ||||||||||||||
| 16 | ||||||||||||||
| 17 | ||||||||||||||
| 18 | ||||||||||||||
| 19 | ||||||||||||||
| 20 | ||||||||||||||
| 21 | ||||||||||||||
| 22 | ||||||||||||||
| 23 | ||||||||||||||
| 24 | ||||||||||||||
| 25 | ||||||||||||||
| 26 | ||||||||||||||
| 27 | ||||||||||||||
| 28 | ||||||||||||||
| 29 | ||||||||||||||
| 30 | ||||||||||||||
| Total wt. | ||||||||||||||
| Min. | ||||||||||||||
| Max. | ||||||||||||||
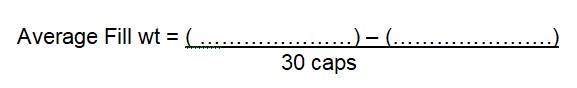
Limit: ± 7.5 %—————–mg to ——————————–mg
Limit: ± 10 %—————–mg to ——————————–mg
INFERENCE:
————————————————————————————————————————–
————————————————————————————————————————–
————————————————————————————————————————–
————————————————————————————————————————–
————————————————————————————————————————–
Checked by:
Date:
| 8.0 | DEFICIENCY AND CORRECTIVE ACTION(S) REPORT(S): |
| The following deficiency was verified and corrective actions were taken in consultation with the validation team. |
| Description of deficiency: |
| Corrective action(s) taken: |
Reviewed By:
Date:
9.0 Annexure (s):
| Sr.No. | Annexure No. | Title of Annexure |
| 10.0 | PERFORMANCE QUALIFICATION FINAL REPORT: |
| 10.1 | SUMMARY: |
| 10.2 | CONCLUSION: |
10.3 FINAL REPORT APPROVAL:
Trial, It has been verified that all tests required by this report are completed, reconciled, and attached to this protocol or included in the qualification summary report. Verified that all amendments and discrepancies are documented, approved, and attached to this protocol.
The signature in the block below indicates that all items in this qualification report of the AF – 25 (Modal Name) Automatic Capsules filling machine have been reviewed and found to be acceptable and We have satisfactorily resolved all variations or discrepancies. The equipment can be taken for production (✅).
| NAME | DESIGNATION | DEPARTMENT | SIGNATURE | DATE |
| PRODUCTION | ||||
| QUALITY CONTROL | ||||
| QUALITY ASSURANCE |
Related Topics:
- Auto Coater Installation Qualification Protocol/ Annexures
- Auto Coater Operational Qualification Protocol/ Annexures
- Auto Coater Performance Qualification Protocol
- Blender Installation Qualification Protocol/ Annexures
- Blender Performance Qualification Protocol/ Annexures
- Blister Machine Operation Qualification Protocol/ Annexures
- Design Qualification in Pharmaceutical Industry
- Design Qualification of Electric Stacker
- Design Qualification Protocol for Vertical Laminar Reverse Flow
- Installation Qualification Protocol for Automatic Capsule Filling Machine
- Installation Qualification Protocol for Blister Packing Machine with Annexures

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
