Validation of the water system is important in the pharmaceutical industry. Water is extensively used in the manufacturing process at various stages. Throughout manufacturing, water is consistently generated and distributed, making it unusable to test and analyze every quantity used in production.
To ensure consistent quality standards, it’s essential to validate the treatment, generation, storage, and distribution processes. Therefore, there are three phases in total for water system validation.
Related: 4 Types Process Validation, Pharmaceutical
Water System Validation Lifecycle:
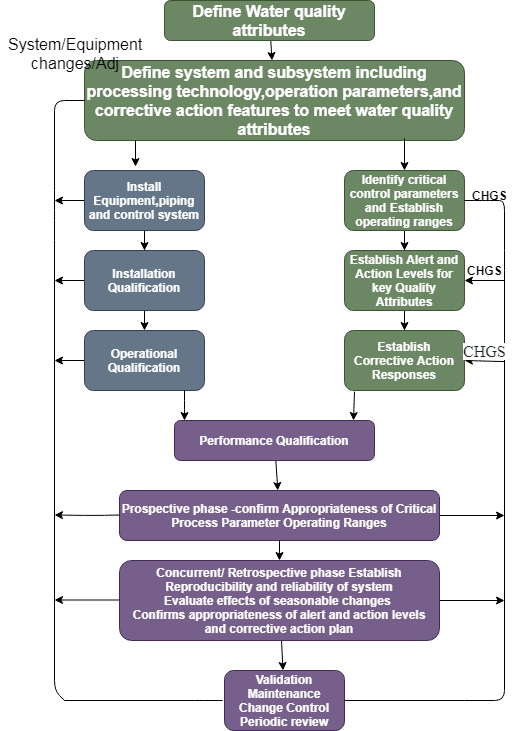
3 Types of Water System Validation
- Water System Validation Phase 1
- Water System Validation Phase 2
- Water System Validation Phase 3
Water System Validation Phase 1:
The time period for phase 1 shall be 2 to 4 weeks (14 days minimum) to Examine the system thoroughly. During this time, the operation must operate continuously without fail or performance modification. Before starting Phase 1, The challenge test should be done for the auto Dump valve and hold Time study. During the Phase 1 validation drain Time study is also to be done. The following should be included within the testing approach:
- Verify its quality by sampling daily from sampling Points.
- Take the sample stepwise after each step of purification.
- Take the daily samples from the point of use of water.
- Develop suitable operating ranges.
- Develop a maintenance procedure and operating cleaning sanitization.
- Demonstrate the production and delivery of product water of the required quantity and quality.
- Use and refine the SOP for operation, maintenance, sanitizing, and troubleshooting.
- Verify provisional alerts and action levels.
- Develop and refine the test-failure procedure.
Related Topic: Cleaning Validation Protocol for Pharmaceutical Equipment
Water System Validation Phase 2:
In Validation Phase 2, a period of 2 to 4 weeks (30 days) is dedicated to conducting additional intensive monitoring. The sampling plan should remain consistent with Phase 1. During this phase, water can be utilized for the production process.
- It shows that a demonstration should be carried out for the consistent production and delivery of water of the required quantity and quality. When the system is operated as per the standard operating procedure.
Water System Validation Phase 3:
After completion of Phase 1 and Phase 2, Phase 3 is typically run for one year. During this phase, water can be used for manufacturing purposes.
- During Phase 3, the sample locations, sampling frequencies, and therefore the test should be reduced to normal routine patterns that shall be supported by established procedures proven during Phase 1 and Phase 2.
- Ensure that seasonal variations are evaluated.
- After completing Phase 3 of the qualification program for the water purification unit system, we should conduct a systematic review. After this review, let’s create a routine plan based on Phase 3.
Monitoring should involve a mix of online instrument monitoring for parameters like flow, pressure, temperature, conductivity, and total TOC, along with specific offline testing for physical-chemical and microbial characteristics.
Related Post: Difference between Validation, Calibration, and Qualification in Pharma
Sampling and Testing:
Offline samples should be taken from the point of use and specific simple points. Sample from the point of use should be taken in a similar way to that adopted when the water is being used in service.
Tests should be done for the determination of conductivity, PH, heavy metals, nitrates, total organic carbon (TOC), total viable count, presence of the specific pathogen, and endotoxin according to pharmacopeia specifications has been satisfied. Monitoring data should be subjected to trend analysis.
Revalidation of the Water System:
Revalidation of the water system should be carried out if there is a major change in the system, the addition or removal of any loop from the system, a change of location, system modification, or any other reason that affects the water quality.
Conclusion:
So, if you are establishing a new facility, you will undoubtedly want assistance with water system validation. so for that validation authority should be approved by state drug control and CDSCO, under the Drugs and Cosmetics Act. Also, validation authority must be approved by state drug control and CDSCO. Furthermore, the Central Drugs Standard Control Organization, India’s national regulatory organization for pharmaceuticals and medical devices, has accepted the validation authority.
FAQs
Ans: It is important that the quality of water should be specific for product quality. Low quality of water can lead to product degradation, contamination, loss of product, and profit.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
