Preparation and Standardization of 0.05 M EDTA Solution by using disodium edetate and Eri Chrom Black T as an indicator in Pharmaceutical Labs.

EDTA, which stands for Edathamil, or Ethylene diamine tetra acetic Acid, is a well-known substance used in managing and treating heavy metal toxicity.
Preparation of 0.05 M EDTA Solution:
To prepare a 0.05 M EDTA Solution, start by carefully measuring around 18.60 grams of disodium edetate and transferring it into a 1000 mL (1-liter) high-quality volumetric flask. Next, dissolve the disodium edetate in enough purified water and fill up the flask with purified water.
Standardizing the 0.05 M EDTA Solution:
Method 1:
- Precisely weigh about 0.10 grams of calcium carbonate (CaCO3) and put it into a conical flask.
- Dissolve it in 3.0 mL of diluted hydrochloric acid (10% v/v HCl) and 10.0 mL of purified water.
- Boil the solution for approximately 10 minutes. Let it cool, and then dilute it to 50.0 mL with purified water (free from CO2).
- Use a dropper to add a few drops of Eri Chrom Black T indicator.
- Titrate the solution with the 0.05 M disodium edetate (EDTA) until you reach the endpoint.
- Keep a record of the volume of disodium edetate solution used during titration.
- Repeat the same procedure two more times.
Method 2:
- Take around 0.40 grams of granulated zinc (Zn) and gently mix it with 12 milliliters of diluted hydrochloric acid (HCl) and 0.05 milliliters of bromine water.
- Heat it a bit to make sure the excess bromine evaporates. Let it cool and then add enough water to make a total of 200.0 milliliters.
- Transfer 20.0 milliliters of this solution into a special flask and almost balance its acidity by adding 2M sodium hydroxide (2M NaOH).
- Fill it up to about 150.0 milliliters with purified water. Add some ammonia buffer with a pH of 10.0 to dissolve any solid, and put in an extra 5.0 milliliters.
- Finally, mix in 50.0 milligrams of mordant black-II mixture and titrate it with the disodium edetate solution until the solution turns green.
Calculation:
- Every milliliter of 0.05M disodium edetate is equal to 0.00327 grams of Zn.
- Do this molarity measurement three times, and then find the average (the results shouldn’t differ by more than 0.2%).
- Keep the solution standardized, ideally every 15 days.
Molarity Formula:
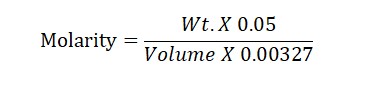
Validity & Precautions of EDTA Solution:
- The solution is good for 30 days from when it’s prepared, and it’s best to re-standardize it every 15 days or when necessary.
- cautious: Check for any changes in the solution’s appearance, like color changes, fungal growth, or sediment.
Preparation of 0.1 M disodium edetate:
Learn about: Preparation and Standardization of 0.01 M EDTA
Read More:
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl
FAQs
To make sure our EDTA solution is just right, we perform a test called standardization. This helps us figure out exactly how strong the solution is.
We use a special indicator called Eriochrome Black T. It’s like a color-changer. When we do the test with EDTA, this indicator goes from blue to pink, and that’s how we know what’s going on.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
