Aim: To Prepare and standardize 0.05 M Iodine Solution by using As2O3
Name: 0.05 M Iodine solution
Ingredients Required for Preparation and Standardization of 0.05 M Iodine Solution:
- Iodine
- Potassium Iodide
- Arsenic Trioxide
- Hydrochloric Acid
- Sodium Hydroxide
- Methyl Orange
- Sodium Bicarbonate
- Starch Solution
How to Preparation and Standardization of 0.05 M Iodine Solution
To Prepare this solution, you’ll need to follow these steps:
- First, take about 14 grams of iodine.
- Then, dissolve it in a solution containing 36 grams of potassium iodide in 100 milliliters of distilled water.
- Add 03 drops of hydrochloric acid.
- Dilute the solution with more distilled water until you have a total of 1000 milliliters.
How to Standardization of 0.05 M Iodine Solution
To check the strength of the solution, follow these steps:
- Accurately weighs about 0.15 grams of arsenic trioxide, which has been dried at 105 degrees for an hour.
- Dissolve it in 20 milliliters of 1M sodium hydroxide by warming it if needed.
- Dilute this with 40 milliliters of distilled water.
- Add 0.1 milliliter of methyl orange solution and slowly add dilute hydrochloric acid until the yellow color changes to pink.
- Next, add 2 grams of sodium bicarbonate, dilute with 50 milliliters of distilled water, and add 3 milliliters of starch solution.
- Titrate this with 0.05 M iodine until a permanent blue color is produced.
- Keep in mind, that each milliliter of 0.05 M iodine is equivalent to 0.004946 grams of As2O3.
Reaction
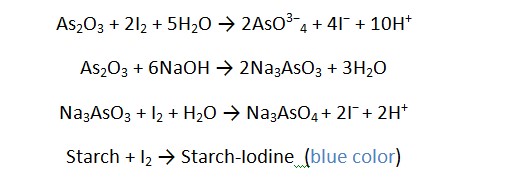
How to Calculate 0.05 M Iodine Solution
To calculate the strength, use this formula:
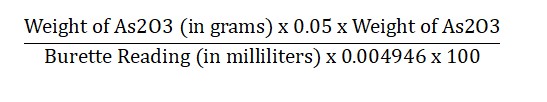
Reference: Indian Pharmacopoeia
Read More:
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
