Preparation and Standardization of 0.1 M KOH Solution by using HCL and phenolphthalein as an indicator in Pharmaceutical Labs.

Aim: To Prepare and measure 0.1 M KOH solution with HCl.
Materials and Reagents Requirements:
Glass: A burette, burette stand, conical flask, volumetric pipette, beaker, volumetric flask, funnel, glass rod, and a wash bottle.
Chemicals: Potassium hydroxide (KOH), hydrochloric acid (HCl), and phenolphthalein indicator.
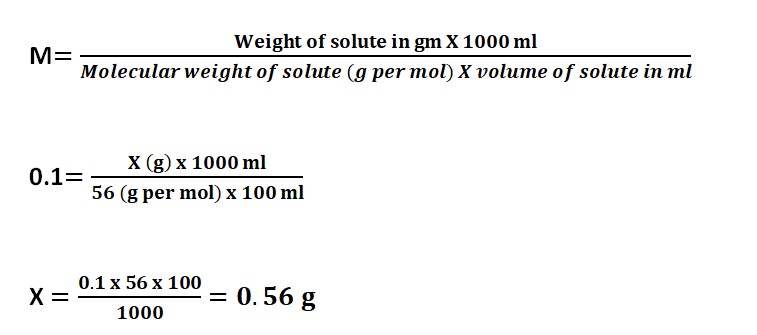
Preparing 0.1 M KOH Solution:
- Take 5.6 grams of KOH and put it in 500 ml of pure water.
- Once it dissolves completely, add more water to make it 1000 ml.
Now the question arises is, How much amount of KOH is required to prepare 0.1 Molar KOH solution in 100 ml?
Let’s Understand it by using the formula:
Molarity=n/v
Whereas, n= no. of moles
v= Volume of solution in a liter, 1 Liter is equal to 1000 ml.
Standardization of 0.1 M KOH Solution
- Clean and dry all glass tools properly.
- Rinse the burette with water, then rinse it with some titrant solution.
- Put the titrant solution in the burette from a clean, dry beaker using a funnel.
- Remove any air bubbles and set the reading to zero.
- Pour 20.0 ml of the solution and add 0.1 M HCl with 0.5 ml of phenolphthalein as an indicator.
- When it turns violet to blue, that’s the endpoint.
- Repeat this process three times for accuracy.
- Record the burette readings, calculate the average, and find the KOH solution’s strength.
- 1 ml of 0.1 M HCl equals 0.005611 g of KOH.
Reference: Indian Pharmacopeia
Read More:
- 0.1 M Sodium Nitrite Preparation and Standardization
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
