Aim: Preparation and Standardization of 0.1 M Silver Nitrate (AgNO3) solution by using sodium chloride in Pharmaceutical Labs.
Name: 0.1 M Silver Nitrate (AgNO3)

Reagents used to Prepare and Standardization of 0.1 M AgNO3 :
- Silver Nitrate (AgNO3)
- Acetic acid (CH3COOH)
- Sodium chloride (NaCl)
- Methanol (CH3OH)
- Eosin Y solution (indicator)
Glassware: Volumetric Flask, measuring cylinder, Burette, Conical Flask, beaker, and Pipettes.
Principle:
In the lab, we used a process where we mixed silver nitrate with sodium chloride. This causes a white, lumpy substance called AgCl and sodium nitrate. We know the reaction is complete when the color changes from a yellow-green to a pinkish-purple. This color change happens because of a special chemical called Eosin Y, which reacts with extra silver ions. We do this experiment in acidic conditions, with a pH of about 2, and it should be done in the dark.
How to Prepare 0.1 M Silver Nitrate:
Dissolve 17.0 grams of Silver Nitrate in enough double distilled water to make 1000 milliliters.
How to Standardization 0.1 M Silver Nitrate:
- Precisely weighs about 0.1 grams of sodium chloride, which has been dried at 110 degrees for 2 hours.
- Dissolve it in 5 milliliters of distilled water.
- Add 5 milliliters of acetic acid, 50 milliliters of methanol, and 0.15 milliliters of eosin Y solution.
- Stir it, preferably using a magnetic stirrer, and titrate it with the silver nitrate solution.
- Each milliliter of 0.1 M silver nitrate is equal to 0.005844 grams of NaCl.
Reaction:
NaCl + AgNO3 → AgCl+ NaNO3
Calculations:
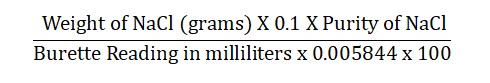
Reference: Indian Pharmacopoeia
Read More:
- 0.1 M Sodium Nitrite Preparation and Standardization
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
