Preparation and Standardization of 0.1 M Sodium Nitrite (NaNO2) by using Sulphanilic acid in Pharmaceutical Analysis.

Chemical Name: 0.1 M Sodium Nitrite (NaNO2)
Reagents:
1.0 Sodium nitrite
2.0 Sulphanilic acid
3.0 Potassium bromide
4.0 2M Hydrochloric Acid
5.0 Starch iodide paper
Preparation of 0.1 M Sodium Nitrite
To make this solution, take 7.5 grams of sodium nitrite and dissolve it in enough distilled water to make 1000 milliliters of the solution.
Standardization of 0.1 M Sodium Nitrite
- To check the strength of the solution, take 0.3 grams of sulphanilic acid and dissolve it in 50 milliliters of 2 M hydrochloric acid.
- Then, add 3 grams of potassium bromide and cool it using ice.
- Now, you can titrate this solution using the sodium nitrite solution and use starch iodide paper to know when it’s ready.
- Each milliliter of 0.1 M sodium nitrite is equal to 0.01732 grams of C6H7NO3S.
Calculation:

Burette Reading (B.R.) is the volume in the burette.
Preparation and Standardization of 0.1 M Sodium Nitrite by using Sulfanilamide in Pharmaceutical Analysis.
Aim: Our goal is to make and measure a 0.1 M sodium nitrite solution using sulfanilamide.
Materials: Glass equipment: Burette, burette stand, flask, pipette, beaker, flask, funnel, glass rod, and a wash bottle.
Chemicals: Sulfanilamide, hydrochloric acid, potassium bromide, and sodium nitrite.
Making of 0.1 M sodium nitrite solution:
Take 7.50 grams of sodium nitrite and dissolve it in 800 milliliters of distilled water. Once it’s fully dissolved, bring the volume up to 1000 milliliters.
Titration process:
- Clean and dry all glassware as per the SOP given.
- Before using the burette, rinse it with distilled water and then with a bit of the solution you’ll use for titration. This makes sure the burette contains the right solution, not something else.
- Fill the burette using a funnel from the unknown stock solution of the titrant.
- Remove air bubbles and set the reading to zero.
- In a 50.00 milliliter solution of 2M hydrochloric acid, dissolve 0.3 grams of sulphanilamide.
- Add 3.00 grams of potassium bromide, cool it on ice, and titrate it with the 0.1 M sodium nitrite solution you prepared until you reach the endpoint, which is determined with a special measurement.
- For accurate results, do the titration three times.
- Make sure to note down the burette readings.
- Find the average of these readings and calculate the strength of the sodium nitrite solution.
Calculations:
1 milliliter of 0.1 M sodium nitrite is equal to 0.01722 grams of sulphanilamide.
Molarity (M) =
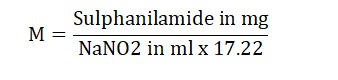
Result: The strength of the sodium nitrite solution we prepared was found to be _ M.
Reference: Indian Pharmacopoeia”
Read More:
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl
FAQs about 0.1 M Sodium Nitrite Solution
0.1 M Sodium Nitrite is a chemical solution that contains sodium nitrite at a specific concentration.
The main ingredients are sodium nitrite, sulphanilic acid, potassium bromide, 2M hydrochloric acid, and starch iodide paper.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
