Preparation and Standardization of 0.1 M Sodium Thiosulfate (Na2S2O3) solution in pharmaceutical labs.

Aim: 0.1 Molar Thiosulfate (Na2S2O3) solution Preparation and Standardization
Materials/Reagents:
1.0 – Sodium Thiosulfate
2.0 – Sodium Carbonate
3.0 – Potassium Bromate
4.0 – Potassium Iodide
5.0 – Hydrochloric Acid
6.0 – Starch Solution
Principle:
Principle: This method uses a standard process called redox titration with iodometric techniques. It involves using potassium bromate as a substance that makes the reaction happen.
First, we take a specific amount of potassium bromate, which is known to be precise and dissolve it in water. Then, we make the solution up to 250 milliliters using more water.
After that, we take 50 milliliters of this solution and add 20 grams of potassium iodide (KI) along with 30 milliliters of a strong acid called 2M HCl. This mixture produces iodine.
To find out how much iodine is produced, we use a solution of sodium thiosulfate along with a substance called starch as an indicator. We keep adding the thiosulfate solution until the blue color in the mixture disappears.
The chemical equation for this reaction is:
KBrO3 + 6KI + 6HCl → KBr + KCl + 3I2 + 3H2O.
Preparation of 0.1 M Sodium Thiosulfate
- Mix 24.8 grams of sodium thiosulfate and 0.2 grams of sodium carbonate in 500 ml of pure distilled water without carbon dioxide.
- Add more distilled water to make it a total of 1000 milliliters.
Standardization of 0.1 M Sodium Thiosulfate
- Dissolve 0.200 grams of potassium bromate accurately in enough distilled water to get 250.0 milliliters of a solution.
- To 50.0 milliliters of this solution, add 2 grams of potassium iodide and 3 milliliters of 2M hydrochloric acid.
- Slowly add the sodium thiosulfate solution with a starch solution as an indicator until the blue color disappears.
- Each milliliter of 0.1M sodium thiosulfate is equal to 0.002784 grams of potassium bromate.
- Make sure to check the strength of the solution regularly.
Calculation:
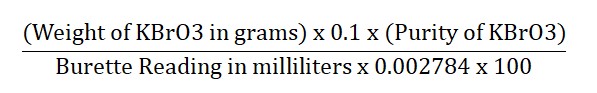
Source: Indian Pharmacopoeia.
Read More:
- 0.1 M KOH Solution Preparation and Standardization
- 0.1 M Sodium Nitrite Preparation and Standardization
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
