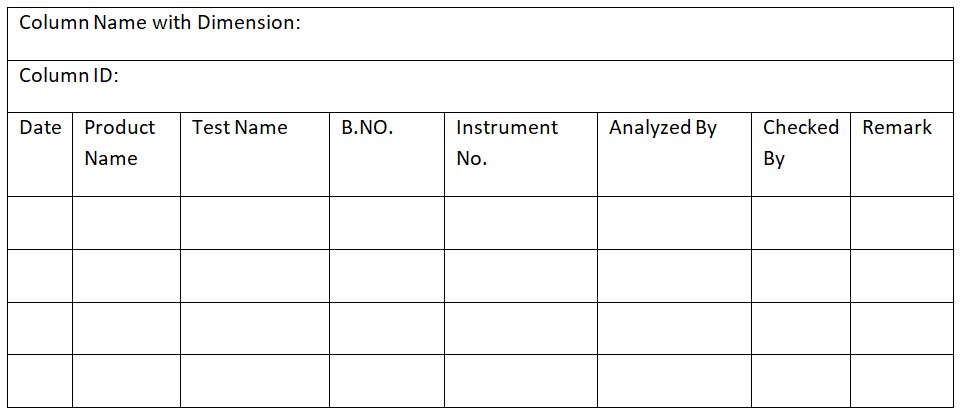
SOP for Operating and Validating a Biosafety Cabinet
Standard Operating Procedure for Operating and Validating a Biosafety Cabinet 1.0 Objective: The purpose of this procedure is to outline how to use and verify the functionality of a Biosafety Cabinet. 2.0 Scope: This procedure covers the usage and internal validation of a Biosafety Cabinet. 3.0 Responsibilities: Microbiologist: Responsible for using, validating, and maintaining the … Read more