Understand and Differentiate them FDA Form 483 and Warning Letters:
FDA Form 483 is like a detailed report card that talks about what people saw when they visited the manufacturing site. These people work for the FDA, and they write down everything they notice in a special order based on how important it is. Each thing they write down is clear, specific, and really matters. And just so you know, the FDA persons who do this have extra training to understand all of it.

An FDA Form 483, is a report, it does not contain any observations of questionable, or unfamiliar significance at the time of the inspection of a specific organization. it only reports on familiar objectionable conditions that are observed during the inspection. even if it can’t report other objectionable conditions, which didn’t notice during the inspection. The company/Firm is only responsible for correcting the objectionable conditions that are noticed after the completion of the inspection on the due date.
How to Handle FDA Form 483?
Once the FDA inspector finishes the inspection at the manufacturing site, the quality person or management of the place should sit down with the FDA inspector and talk about, what the inspector found. They should really try to fix any problems before the inspector goes away. If things get confusing or there are mistakes in what was said, it’s a good idea to ask the inspectors for help.
It’s important to figure out if the people from the FDA are feeling positive or negative. Sometimes they might feel negative for different reasons or even for no reason at all. If something is unclear during their observations, don’t be afraid to ask the inspector questions.
If you’re confident, give it a shot and try to convince the inspector. You can use related information to make your point about what was seen during the inspection. Your ability to convince might even help remove some of the things listed in the FDA Form 483.
The point of an FDA Form is to let the management of the company know about any problems. They sit down and talk about the FDA Form 483 with the management after the inspection is done. The management is supposed to come up with a plan to fix things by a certain date.
You have to reply to the FDA Form 483 within 15 days, or else the Regulatory persons won’t pay attention to what you said about the problems in your company. The FDA Form 483 isn’t the final say. It’s taken into account along with a written report called the Establishment Inspection Report. They look at all the inspection reports collected from the manufacturing site, and the answers the company gives, and then they decide what to do next.
If you answer the FDA Form 483 on time and explain things well, you might avoid getting a serious Warning Letter.
FDA Form 483 Details:
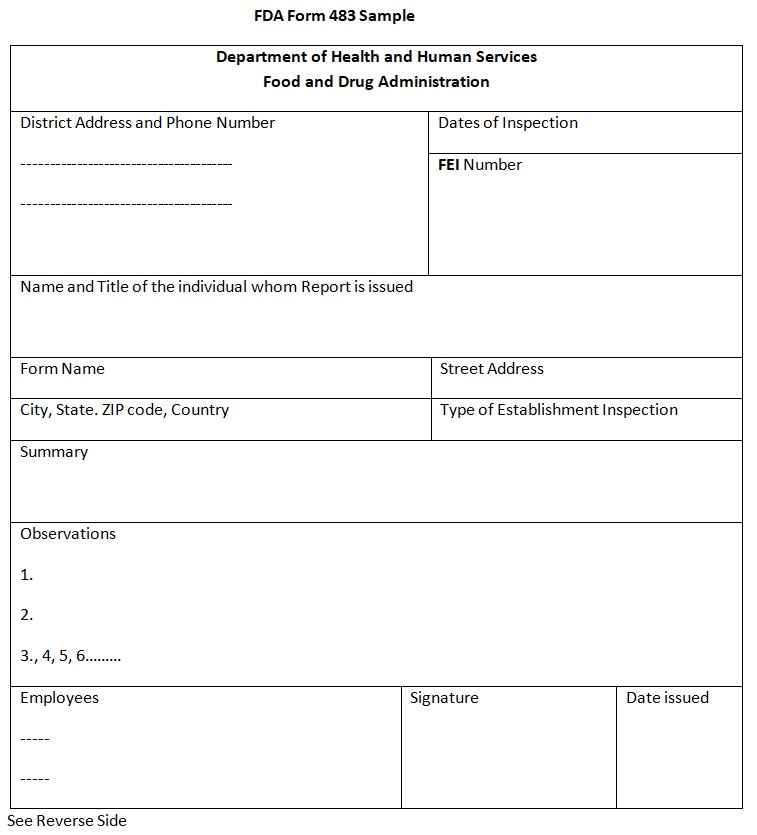
1. Header Information
This part has the following details:
- Address and phone number of the FDA district office.
- Dates when the inspection happened.
- The FDA Establishment Identification Number (FEI) of the facility.
- Name and title of the person who got the 483.
- Address of the place that got inspected.
- A quick description of what type of place it is.
2. Observations
In this section, you’ll find all the observations written by FDA investigators. The number of investigators can be one to three, as per FDA rules. Remember, this section isn’t the final decision from the agency. You can talk to the FDA investigator about what they observed, and you can also share your plans for fixing things. If you have any questions, you can reach out to the FDA using the contact info in the header.
3. Notes/Summary
When the final discussion happens, the 483 is explained to the folks who manage the place. If they don’t want any notes, they won’t have them. Notes might be at the end of each page or the last page of the observations where the investigators usually sign. In 1997, the FDA made a policy about notes for medical device inspections.
4. Signatures
On the first and last page, you’ll find the FDA investigator’s signatures. Other pages just have their initials. All investigators’ names are printed and signed, and the date when the 483 was issued is written here. The FDA investigator’s job title might also be mentioned.
5. Back Side
On this side, you’ll see some instructions about Food, Safety, and cosmetic devices. There are also directions for FDA investigators about their work at the place they inspected. All the instructions here are based on specific laws like the Federal Food, Drug, and Cosmetic Act, Section 704(b).
Other FDA Forms Explain:
You might have heard about the FDA 483 form, but did you know that there are other related forms like FDA 481, 482, and 484? Let’s check out Below:
FDA 481:
The FDA 481 form is used by the US Food and Drug Administration (FDA) during inspections of various facilities, such as those that handle foods, supplements, medical devices, and other items. This form helps the FDA to determine if these facilities are following proper safety standards for hygiene, pest control, packaging quality control, and food handling.
Because it contains information about the product and its development goals, this form is especially important for companies developing novel drugs or medical devices. Before submitting Form 481, companies are required to first register with the FDA.
FDA 482: Written Notice of Inspection
When the FDA conducts an inspection, they may do so for various reasons, such as routine checks or in response to a reported issue. The investigator will arrive at the facility with credentials and a “Notice of Inspection” (FDA Form 482).
The FDA Form 482 is an official document filled out by FDA investigators and given to the responsible person at the site being inspected. It marks the start of the inspection.
FDA 483: Inspectional Observations
After an inspection, if the FDA investigator observes conditions that could be violations of FDA regulations, they issue a Form FDA 483 to the management of the inspected firm. This form lists the observations made during the inspection.
Form 484: Receipt for Samples
At the end of an inspection, if any samples were obtained, the FDA issues Form 484. This document describes the samples collected and is given to the same person who received the FDA 482.
Warning Letter:
Once the FDA Form 483 has been given out, the next step could be a Warning Letter. This is when the FDA decides to send a formal letter to the place where medicines are made. This usually happens if the FDA finds some serious problems with how the products are made. These problems could affect the quality of the products. The Warning Letter comes from important people at the FDA, who carefully review all the things that were seen during the inspection, especially the quality of the products.
After the FDA Form 483 is given and the inspection is done, the FDA might send a Warning Letter to the place that was inspected. If there’s a big problem with the product quality, the higher-ups at the FDA will write a Warning Letter. They include proof and detailed explanations about what was found. The top officials at the FDA really care about product quality and won’t make any compromises.
It’s important to reply to a Warning Letter within the given time because if you take too long, they might stop products from coming into the country. Sometimes you can ask for more time to explain things better. Most Warning Letters from the important people at the FDA are about problems with product quality or how things are made.
You can easily find both FDA Form 483 and Warning Letters on their website. These things are put up there right away for everyone to see on fda.gov. Anyone can access them.
Types of Warning Letters:
General FDA Warning Letters
These are given when the FDA sees that a manufacturer has broken important rules. This could be because the way things are made is not good, there are problems with what the product can do, or the instructions for using it are wrong. These letters are sent out when there are big issues. The company has to fix the problems mentioned in the letter and tell the FDA how they plan to fix them. The FDA and the company might talk more about this, and what happens next can change based on their discussions.
See the List of Current Warning letters (FDA Warning Letters List)
Tobacco Retail Warning Letters
These are sent when the FDA finds problems connected to selling tobacco items. If there are issues with how these products are sold, these letters could be given.
Drug Marketing and Advertising Warning Letters
These are given if there are problems with how a drug is promoted. If the advertising isn’t following the rules, the FDA can send out these letters.
Tobacco Retail Warning Letters
Shops that sell tobacco products sometimes face checks to make sure they’re following the rules from the Family Smoking Prevention and Tobacco Control Act and the Regulations that keep tobacco away from kids and teens. These checks also include cigarettes and smokeless tobacco products.
Drug Marketing and Advertising Warning Letters
These letters are collected and grouped by month, and they only talk about how drugs are advertised. Some parts of the letters might be changed to keep private information safe. Letters sent electronically might be involved in illegal activities.
Closing a Warning Letter
The FDA can issue a “close-out letter” after sending a Warning Letter. The FDA looks at the changes the company made in response to the Warning Letter. If the FDA thinks the changes are good, they might send a close-out letter. But if the company didn’t do enough, the FDA won’t send one. The FDA needs to check if the changes are real. They hope the issues are fixed and they can check during a follow-up visit.
If there are still problems that can’t be fixed, no “close-out letter” will be given. The FDA might inspect again in the future to see if things are better, and they might take action without warning if needed.
Read other’s Articles:
- Nonconformity During Audit and its Types with Examples
- Pharmaceutical Quality System (PQS), ICH Q10 Guidelines
- Change Management System in Pharmaceuticals
- Complete Overview of ISO CleanRoom Classification and Risk Assessment
- SCADA System in Pharmaceuticals and Their Role
- Difference Between Classified and Non-Classified Areas in Pharmaceuticals
- Basic Difference Between BMR and eBMR/eBPR in Pharmaceuticals
- Good Laboratory Practice (GLP) in Pharmaceutical
- What is GMP | cGMP | GMP Principle
- ICH Guidelines in Pharmaceutical (updated)
- Accelerated stability testing (study) Important Questions
- ALCOA to ALCOA Plus and Data Integrity
Reference:
- FDA- https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/compliance-actions-and-activities/warning-letters
- FDA-https://www.fda.gov/media/162162/download (Understand the Form 483 Process and Timeline)
- FDA- https://www.fda.gov/media/157073/download (USFDA Form 483)

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].

