Before beginning an on-site audit, the following Check List for Visitors points should be considered:
- Make a plan.
- Review previous audits and note any potential problem areas or items flagged for corrective action.
- Learn about the type of product produced here and how it is arranged by personnel and function if unfamiliar with the facility.
Using the WHO-GMP Document Checklist can help you understand the requirements during visits to the premises.
Document Check List for Visitors
- Site Master File.
- Air Quality Manuals.
- Water Quality Manual.
- All process-related SOPs.
- Standard Testing Procedures (STPs) and Specifications for Raw Material, Packing Material, Intermediate, and Finished Products.
- Market Complaints Record.
- Calibration Record.
- Process Validations report for 3 Batches.
- Analytical Method Validations.
- Cleaning Method Validations.
- DQ/IQ/OQ/ PQ.
- Internal Quality Audit Reports.
- Medical Checkup Record of Employee.
- Master Formula Records.
- Batch Manufacturing Records.
- Batch Packing Records.
- List of Machines and Equipment for Production and QA/QC(QU- Quality Unit).
- List of Competent Technical Staff.
- Latest FDA Approved Plan.
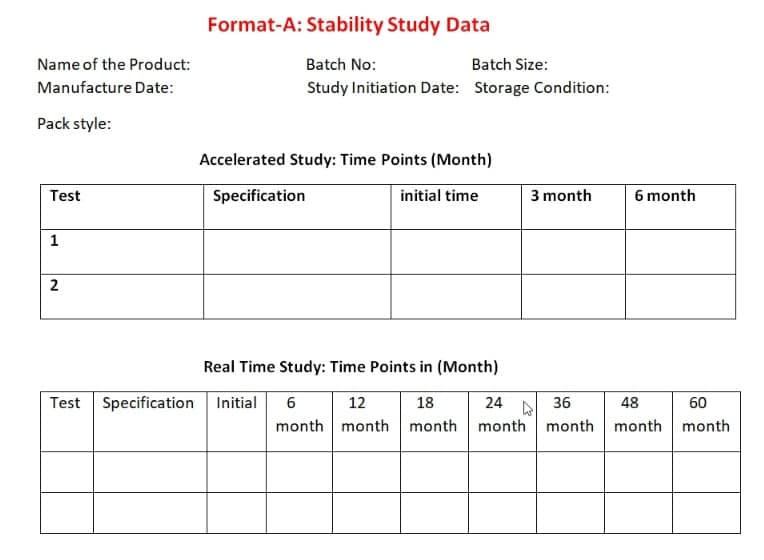
- Short Term, Long Term, and Photostability (Zone wise).
- Stability study evaluation (accelerated and real-time) for three batches, including batch size, batch number, product name, expiry dates, stability study condition (accelerated/real-time), drug name, and so on (as per Format-A) (At least a six-month timeframe for Accelerated Stability data and a year for other data.) At the time of initial submission, real-time stability data must be submitted.)
- Approved Vendor List and Vendor Evaluation Record.
- Employee Training Record.
- Pest Control, Premises Maintenance Record (Sanitation, Cleaning ).
- Cloth Washing and Laundering Record.
- Preventative and Break Down Maintenance Record.
- All other Records as per WHO GMP Guidelines.
- Must include an Annual product quality review (APQR) and Growth promotion test evaluation.
- Preservative efficacy testing.
- Risk analysis and critical control point documentation
- Rule 158B of the Drugs and Cosmetic Rules, 1945, requires proof of safety and effectiveness.
- last inspection Date
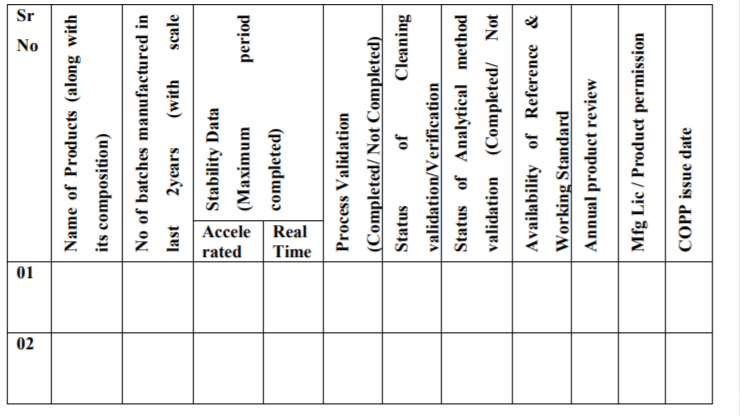
Format-B: Product summary sheet
Conclusion:
A Good Manufacturing Practice (GMP) audit checklist is one of the most effective methods available for importers to assess their supplier’s readiness for a WHO-GMP inspection. However, creating a WHO-GMP Document Checklist is not a simple task. It can take a long time to create, execute, and maintain a complete checklist.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
