Preparation and Standardization of 0.05 M EDTA Solution by using disodium edetate and Eri Chrom Black T as an indicator in Pharmaceutical Labs.

EDTA, which stands for Edathamil, or Ethylene diamine tetra acetic Acid, is a well-known substance used in managing and treating heavy metal toxicity.
Preparation of 0.05 M EDTA Solution:
To prepare a 0.05 M EDTA Solution, start by carefully measuring around 18.60 grams of disodium edetate and transferring it into a 1000 mL (1-liter) high-quality volumetric flask. Next, dissolve the disodium edetate in enough purified water and fill up the flask with purified water.
Standardizing the 0.05 M EDTA Solution:
Method 1:
- Precisely weigh about 0.10 grams of calcium carbonate (CaCO3) and put it into a conical flask.
- Dissolve it in 3.0 mL of diluted hydrochloric acid (10% v/v HCl) and 10.0 mL of purified water.
- Boil the solution for approximately 10 minutes. Let it cool, and then dilute it to 50.0 mL with purified water (free from CO2).
- Use a dropper to add a few drops of Eri Chrom Black T indicator.
- Titrate the solution with the 0.05 M disodium edetate (EDTA) until you reach the endpoint.
- Keep a record of the volume of disodium edetate solution used during titration.
- Repeat the same procedure two more times.
Method 2:
- Take around 0.40 grams of granulated zinc (Zn) and gently mix it with 12 milliliters of diluted hydrochloric acid (HCl) and 0.05 milliliters of bromine water.
- Heat it a bit to make sure the excess bromine evaporates. Let it cool and then add enough water to make a total of 200.0 milliliters.
- Transfer 20.0 milliliters of this solution into a special flask and almost balance its acidity by adding 2M sodium hydroxide (2M NaOH).
- Fill it up to about 150.0 milliliters with purified water. Add some ammonia buffer with a pH of 10.0 to dissolve any solid, and put in an extra 5.0 milliliters.
- Finally, mix in 50.0 milligrams of mordant black-II mixture and titrate it with the disodium edetate solution until the solution turns green.
Calculation:
- Every milliliter of 0.05M disodium edetate is equal to 0.00327 grams of Zn.
- Do this molarity measurement three times, and then find the average (the results shouldn’t differ by more than 0.2%).
- Keep the solution standardized, ideally every 15 days.
Molarity Formula:
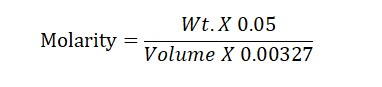
Validity & Precautions of EDTA Solution:
- The solution is good for 30 days from when it’s prepared, and it’s best to re-standardize it every 15 days or when necessary.
- cautious: Check for any changes in the solution’s appearance, like color changes, fungal growth, or sediment.
Preparation of 0.1 M disodium edetate:
Learn about: Preparation and Standardization of 0.01 M EDTA
Read More:
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl
FAQs
To make sure our EDTA solution is just right, we perform a test called standardization. This helps us figure out exactly how strong the solution is.
We use a special indicator called Eriochrome Black T. It’s like a color-changer. When we do the test with EDTA, this indicator goes from blue to pink, and that’s how we know what’s going on.