Preparation and Standardization of 0.1 M Sodium Nitrite (NaNO2) by using Sulphanilic acid in Pharmaceutical Analysis.

Chemical Name: 0.1 M Sodium Nitrite (NaNO2)
Reagents:
1.0 Sodium nitrite
2.0 Sulphanilic acid
3.0 Potassium bromide
4.0 2M Hydrochloric Acid
5.0 Starch iodide paper
Preparation of 0.1 M Sodium Nitrite
To make this solution, take 7.5 grams of sodium nitrite and dissolve it in enough distilled water to make 1000 milliliters of the solution.
Standardization of 0.1 M Sodium Nitrite
- To check the strength of the solution, take 0.3 grams of sulphanilic acid and dissolve it in 50 milliliters of 2 M hydrochloric acid.
- Then, add 3 grams of potassium bromide and cool it using ice.
- Now, you can titrate this solution using the sodium nitrite solution and use starch iodide paper to know when it’s ready.
- Each milliliter of 0.1 M sodium nitrite is equal to 0.01732 grams of C6H7NO3S.
Calculation:

Burette Reading (B.R.) is the volume in the burette.
Preparation and Standardization of 0.1 M Sodium Nitrite by using Sulfanilamide in Pharmaceutical Analysis.
Aim: Our goal is to make and measure a 0.1 M sodium nitrite solution using sulfanilamide.
Materials: Glass equipment: Burette, burette stand, flask, pipette, beaker, flask, funnel, glass rod, and a wash bottle.
Chemicals: Sulfanilamide, hydrochloric acid, potassium bromide, and sodium nitrite.
Making of 0.1 M sodium nitrite solution:
Take 7.50 grams of sodium nitrite and dissolve it in 800 milliliters of distilled water. Once it’s fully dissolved, bring the volume up to 1000 milliliters.
Titration process:
- Clean and dry all glassware as per the SOP given.
- Before using the burette, rinse it with distilled water and then with a bit of the solution you’ll use for titration. This makes sure the burette contains the right solution, not something else.
- Fill the burette using a funnel from the unknown stock solution of the titrant.
- Remove air bubbles and set the reading to zero.
- In a 50.00 milliliter solution of 2M hydrochloric acid, dissolve 0.3 grams of sulphanilamide.
- Add 3.00 grams of potassium bromide, cool it on ice, and titrate it with the 0.1 M sodium nitrite solution you prepared until you reach the endpoint, which is determined with a special measurement.
- For accurate results, do the titration three times.
- Make sure to note down the burette readings.
- Find the average of these readings and calculate the strength of the sodium nitrite solution.
Calculations:
1 milliliter of 0.1 M sodium nitrite is equal to 0.01722 grams of sulphanilamide.
Molarity (M) =
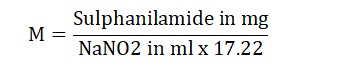
Result: The strength of the sodium nitrite solution we prepared was found to be _ M.
Reference: Indian Pharmacopoeia”
Read More:
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl
FAQs about 0.1 M Sodium Nitrite Solution
0.1 M Sodium Nitrite is a chemical solution that contains sodium nitrite at a specific concentration.
The main ingredients are sodium nitrite, sulphanilic acid, potassium bromide, 2M hydrochloric acid, and starch iodide paper.

