Technology Transfer Checklist For Sending Plant
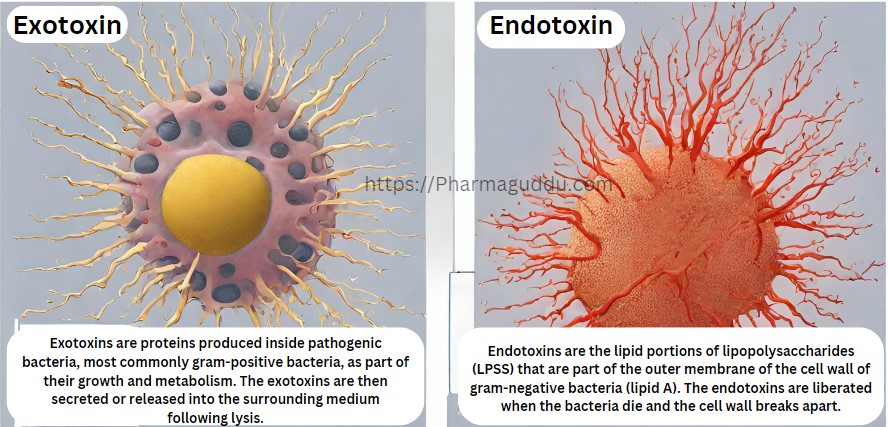
When the process of moving technology including (Chemicals, Production/ Manufacturing, Control, and drug product-related documents) from one place to another, so we can make the same good stuff every time, that’s called technology transfer. Below is the full Checklist of Technology Transfer For Sending the Plant. Project identification No. — Name of Product — Technology … Read more