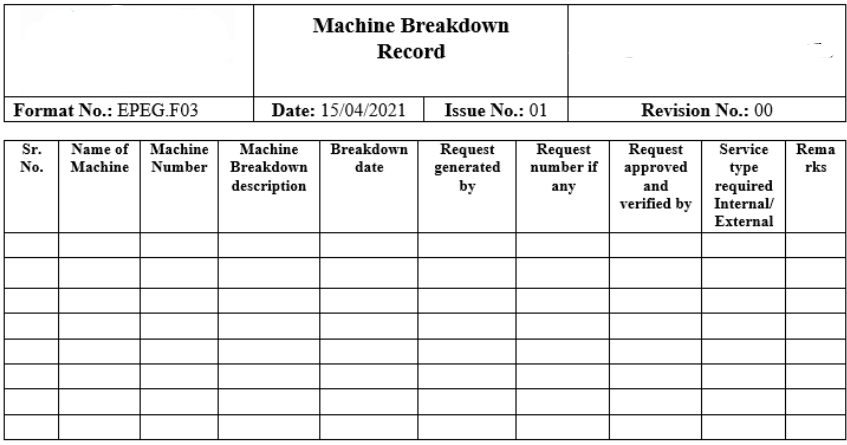
SOP for Operating and Cleaning of Dry Bath Incubator
Here You will learn about Standard Operating Procedure for Operating and Cleaning of Dry Bath Incubator including their Purpose, Scope, Responsibilities, Procedure, Cleaning, Precautions, maintenance, and Various Records. Purpose: To establish a standard operating procedure for operating and cleaning of Dry BathIncubator used in Laboratory. To meet compliance with Good Laboratory Practices. Scope: This standard … Read more