Preparation and Standardization of 0.1 N Perchloric acid (Non-Aquos by using glacial acetic acid and crystal violet solution as indicators in Pharmaceuticals.
Apparatus and Reagents Requirements:
Apparatus: Conical Flask, Volumetric Flask, Reflux condenser, Measuring cylinder, Burette, Pipette and Burette Stand.
Reagents:
- Perchloric acid
- Potassium hydrogen phthalate
- Anhydrous glacial acetic acid
- Crystal violet solution
Preparation of 0.1 N Perchloric acid
- Mix 85 ml of perchloric acid with 500 ml of anhydrous glacial acetic acid and 25 ml of acetic anhydride.
- Cool and make up the volume up to 1000 ml with glacial acetic acid.
- Allow the solution to stand for 24 hours and determine the water content before use.
- If the water content exceeds 0.05% add some more acetic anhydride.
- It also acts as an oxidizing agent so it should be handled with care.
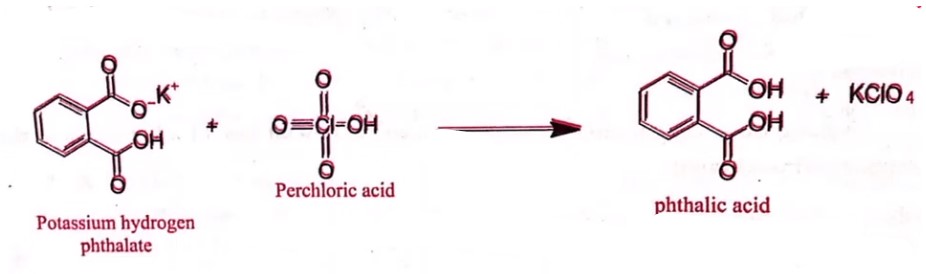
Standardization of 0.1 N Perchloric acid
- Standardization: Weigh accurately about 0.35 gm of potassium hydrogen phthalate previously dried at 120°C and dissolved in 50 ml of glacial acetic acid.
- Add 0.1 ml of crystal violet solution and titrate with 0.1 N perchloric acid until the violet color changes to an emerald green color.
- Each ml of 0.1 N perchloric acid is equivalent to 0.02042 gm of potassium hydrogen phthalate
Calculation:
N1V1=N2V2
0.1 x 15 (Burrete Reading)= N2 x 25 ml (solution Taken)
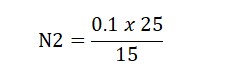
Result: The Normality of Perchloric Acid was found to be…?
Read More:
- 0.1 M KOH Solution Preparation and Standardization
- 0.1 M Sodium Nitrite Preparation and Standardization
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl
FAQs
Ans: because CH3COOH acts as an acid & as well as a base but when we take per choric acid it is a very strong acid so we use acetic acid.

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
