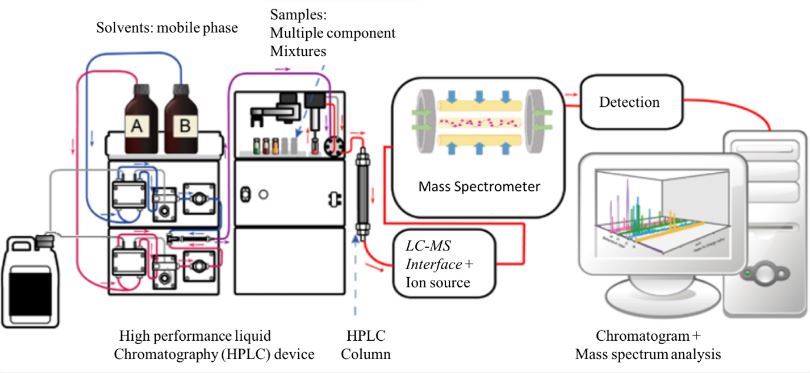
SOP for Cleaning and Operation of conveyor belt and turn table
1.0 Objective: To lay down a procedure for the Cleaning and Operation of conveyor belt and turn table. 2.0 Scope: This procedure is applicable to the Cleaning and Operation of conveyor belt and turn table in Liquid department. 3.0 ResponsibilityOperator, officer – Production Department 4.0 Procedure4.1 Cleaning4.1.1 Cleaning of conveyer belt4.1.1.1 Start the conveyor belt … Read more