Fumigation and fogging are commonly used in pharmaceuticals to control microbial contamination in controlled areas. Fumigation is a gaseous sterilization process that kills microorganisms and prevents microbial growth in the air or on surfaces such as walls and floors.

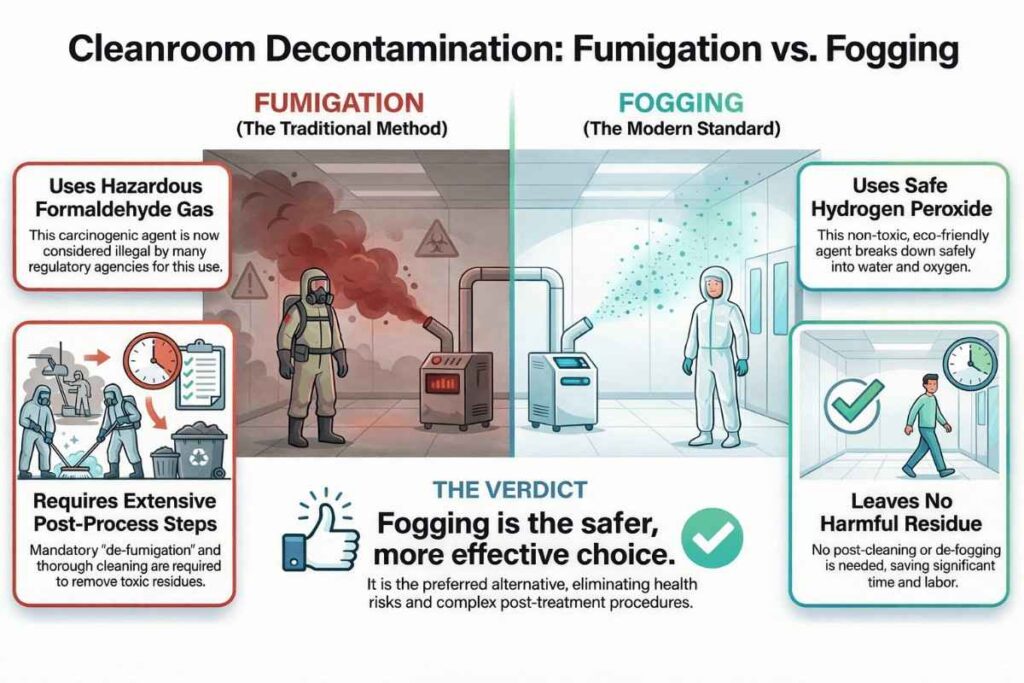
Fumigation agents, such as formaldehyde and potassium permanganate, are often used to control microorganisms. This technique also helps eliminate lizards, cockroaches, cobwebs, rodents, flies, and insects from the area. However, nowadays, the use of formaldehyde and potassium permanganate for fumigation is considered illegal by various regulatory agencies in pharmaceutical industries.
Visual Guide on Fogging vs Fumigation Comparison:
Why is formaldehyde banned in Pharmaceuticals?

The primary reason for banning formaldehyde is its carcinogenic nature and the associated cancer risk, especially for personnel who work with it. Formaldehyde can also cause irritation to the eyes and nose.
Difference Between Fumigation and Fogging?
1. Fumigation:
Fumigation is the process of spraying formaldehyde and potassium permanganate in liquid form to generate sterilizing gas.
Principle of fumigation:
Formaldehyde is chemically represented as H-CHO in its pure form. It exists as a gas at room temperature, with a boiling point of −19 °C. However, at temperatures below 80 °C, it readily undergoes polymerization to form a white solid. One of its significant polymers is paraformaldehyde, an oligomer or short polymer of formaldehyde, which is colorless and releases formaldehyde gas rapidly when heated.
Although formaldehyde has strong bactericidal properties, its penetration power is relatively weak. For an effective fumigation process, the gas must be uniformly spread in the environment where bacteria are present. Therefore, in pharmaceutical industries, fumigation is recommended at temperatures above 22 °C and at 75% relative humidity.
Advantages of fumigation:
- Controls all life stages of microorganisms due to its toxic nature
- Provides rapid microbial control, saving both time and cost
- Easily reaches areas where aerosols and sprays cannot penetrate
- Leaves no residue
Mode of action:
Fumigation works by producing cross-links between proteins that interact with RNA and DNA. Formaldehyde acts as a mutagenic and alkylating agent that reacts with carbonyl, thiol, and hydroxyl groups.
It requires approximately 75% relative humidity to be most effective (acceptable range: 60–80% humidity and temperature around 22 °C), as the gas must dissolve in the moisture film surrounding bacteria.
How to Use Fumigation:
Steam may be used to increase room humidity and temperature to the required levels.
- Use 500 ml of formaldehyde with 1 liter of distilled water for 28 m³ of area for four hours or overnight.
- Use 170 g of potassium permanganate with 500 ml of formaldehyde for 28 m³ of area for four hours or overnight.
These concentrations may be adjusted after proper validation.
2. Fogging:
Fogging involves the use of a mixture of hydrogen peroxide and silver ions. It is widely used in pharmaceutical industries to control microbial contamination in controlled areas.

Advantages:
- Non-toxic, eco-friendly, chlorine-free, and non-mutagenic
- Available in multiple formulations, typically containing 10% hydrogen peroxide with acidic pH and no odor
- Water-miscible and cost-effective
How to Use Fogging:
For an area of 1,000 ft³, prepare a 20% solution by mixing 200 ml of the disinfectant solution with 800 ml of demineralized water. Use a fogger to spray the solution for 30 minutes at a fogging rate of 130 ml per minute. Allow the fog to remain on surfaces for at least one hour.
Mode of action:
Hydrogen peroxide is a powerful oxidizing agent that neutralizes microorganisms and renders them inactive. Virosil is a commercially available product containing 10% hydrogen peroxide. The recommended concentrations are:
- Surface disinfection: 5%
- Instrument disinfection: 10%
- Resin and filter disinfection: 1–3%
- Storage tank disinfection: 5%
- Laundry disinfection: 10%
In the case of hydrogen peroxide and silver ions, the solution is considered safe for personnel. No de-fogging is required after fogging, as residues decompose into water and nascent oxygen. Therefore, there is no need for cleaning or mopping after the process.
Precautions During the Fumigation and Fogging Process
- Stop all operations before starting fumigation or fogging
- Remove all raw materials, finished goods, intermediates, and in-process materials
- Ensure all personnel follow proper entry and exit procedures
- Close all windows and doors; switch off AC and AHU systems
- Display status labels stating “Area under fumigation or fogging – Do not enter” at all entrances
- Inform security personnel and schedule additional security rounds
Cleaning after Fumigation:
After fumigation, clean the area using a 70% v/v IPA solution and lint-free cloths. External parts of equipment should be wiped simultaneously. Floors and drain points should be cleaned as per the relevant SOP titled Cleaning and Sanitization in Pharmaceuticals.
Fumigation should be scheduled after prolonged shutdowns or during weekends before plant start-up.
Validation of Fumigation in Pharmaceuticals:
Validation confirms the effectiveness of fumigation. To prove this, Biological indicator strips containing Geobacillus stearothermophilus ATCC 7953 spores are commonly used. These indicators are marketed under the name BIO NOVA and consist of perforated stainless-steel coupons containing spores.
The strips are placed at various locations, especially critical areas such as corners and behind equipment. After fogging, the strips are incubated in MC20 growth medium at 60 ± 2 °C for 24 hours.
Validation Criteria for Fogging
If the MC20 growth medium changes color to yellow after incubation at 60 ± 2 °C for 24 hours, it indicates microbial growth and ineffective fogging. If no color change is observed, the fumigation or fogging process is considered effective.
De-Fumigation in Pharmaceuticals
De-fumigation is carried out by operating the AHU continuously for several hours without any activity in the area. This process removes residual gases from the air. Equipment cleaning after fumigation is essential to ensure safety and compliance.
Conclusion:
Fogging is a safer and more effective method than fumigation for controlling microorganisms in pharmaceutical environments. It is preferred because it is personnel-friendly, leaves no residues, and does not require cleaning or de-fogging procedures.
Fogging rapidly eliminates bacteria and fungi without the need for extensive safety measures. In contrast, fumigation requires regular monitoring and strict handling precautions. Therefore, fogging is considered the best alternative to traditional fumigation in pharmaceutical facilities.
Related Topics:
- Difference between Humidity and Relative Humidity
- Moisture Content and Loss On Drying (LOD) in Pharmaceuticals
- pH Meter | Principle, Calibration, and Working
- Good Laboratory Practice (GLP) in Pharmaceutical
- High-Performance Liquid Chromatography (HPLC)
- Injectable | Parenteral | sterile preparations, types, Standard test
- Tablet Friability Test Calibration and Specification
- Pharmaceutical Sampling, types, tools ( Guidelines)
- Vernier Caliper measurement and operation in Pharma
- Handling of Laboratory Incidents in Pharma
FAQs
Ans: Due to its carcinogenic properties and associated cancer risks for personnel.
Ans: Operating air handling units continuously removes residue after fumigation.
Ans: Fumigation sprays formaldehyde, while fogging uses hydrogen peroxide and silver ion solution.
Ans: Controls microorganisms at all stages, quickly, easily, and leaves no residue problems.
Ans: Fogging uses non-toxic, eco-friendly agents like hydrogen peroxide and silver ions.
Ans: Stop operations, remove materials, follow entry procedures, close doors, and switch off AC.
Ans: Indicator strips like BIO NOVA with Geobacillus stearothermophilus verify effectiveness after fogging.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].

Appreciable. Very informative.
Good Information. Very and easy to understand